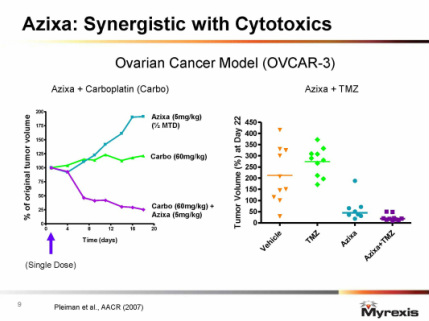
Myrexis MYRX Stock Research - Azixa page
Azixa - Introduction
- aka verubulin aka MPC-6827
- microtubule destabilizing agent to induce apoptosis and VDA. 6/6/11: same class as taxol, vincristine (aka Oncovin) and vimblastine (aka Velbe), but is differentiated because (unlike the above 3 drugs) it is not a substrate for multiple drug resistance (MDR) pumps and therefore freely crosses the blood brain barrier (aka BBB, which primary consists of said MDR pumps)
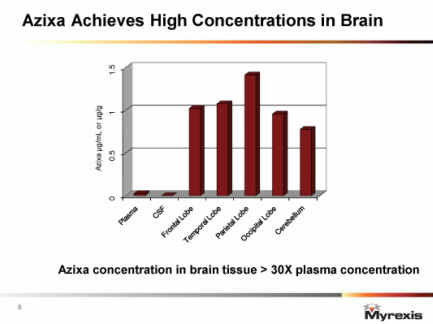
- 6/6/11 webcast: Azixa accumulates in neuronal tissue- hardly any is found in CSF (see figure below). Over 160 pts treated so far and seen no indcation of any neurotoxicity (cognitive or motor function). Drug appears to be benign to normal non-dividing neural cells - only kills dividing cancer cells
- Developing for advanced primary and metastatic tumors w/ brain involvement. High brain penetration (14-30x preferential accumulation vs serum)
- 5/2011 webcast: "In the industry, there has been much focus lately on targeted cancer therapies, but big pharma know these only address small parts of the markets. They therefore seek some more generalized mechanisms with broader applicability. Ask themselves, can you really get away w/ $120k/yr cost for small group of pts?" [I would argue that yes, they can!]
- 2 p1s completed 2007 w/ 66 pts total (6 pts had SD ranging from 5-16 months). Click for first clinicaltrials.gov entry - Click for second clinicaltrials.gov entry.
- Would owe mid-high single digit royalties to Epicept and up to $23m milestones (next milestone would be dosing of first patient in a phase 3 trial).
- Think can afford only single registrational study without partner
- No approved drugs for brain mets.
- have COM patent, exp?? [last issued patent exp 2024].
- Backup oral analog MP443803 in hand but not under active development.
Azixa program in GBM (glioblastoma multiforme)
- Gliblastoma multiforme: [~4000/yr, 15-20% of primary brain tumors, survival 12-18 months, among most vascularized tumors, first line is resection then radiation and temozolomide, fastest route to registration, avastin approved].
- Clinical trial details: A) 2008 initiated open-label p1/2a trial in combo w/ 2nd line chemotherapy.
---Preliminary data presented at ASCO 6/2010: combo was well tolerated - no Azixa dose reduction needed, with 2 PR (lasting 8 and 26 months) and 6SD (range 1-7 months) in 19 pts, ORR 42% using MacDonald criteria, median PFS 2 months.
---Previously had said would release more data late 2010 but that never happened
---Listed as ongoing as of 7/29/11 on clinicaltrials.gov.
- B) 2q2009 initiated open-label p2a monotherapy trial in recurrent GBM.
---Enrolled 56 total pts who received Azixa weekly 3 of 4 weeks, primary endpoint was PFS.
---Avastin (bevacizumab)-experienced cohort data presented 11/2010 at SNO (Click here for PR): 25 third-line pts completed median of 1 cycle, one PR by MacDonald criteria with 80% regression at 12 months, 4 SD. Median PFS 22 days [mean 37 days]. Median OS 94 days (mean 105 days)
---Avastin-naive cohort data presented 6/2011 at ASCO (Click here for PR - Click here for blog post with abstract and my notes - click here to download complete poster): In this group, 31 pts with recurrent GBM who had failed temozolomide but not tx with avastin completed a median of 2 cycles of Azixa therapy, which was well tolerated. 14% of pts were progression free at 6 months, with median PFS of 1.8 months and median OS of 9.9 months. 3 PR's and 7 SD's (median 3.9 months) were observed for a clinical benefit rate of 32%- described as "modest activity". These data are all better than seen in Avastin-experienced patients.
- C) 12/2010 initiated randomized p2b in combo with standard of care (SOC) (NCT01285414)
---Estimate progression in about 6 months on control arm, Part B primary endpoint is PFS at 9 months. OS and correlation of survival with genotype (have not seen any details on this) are secondary endpoints. Primary endpoint of Part A is safety at 14 weeks.
---5/10/11 call: Part A requires evaluating 3-6 pts per group thru 2 cycles of 4 weeks each, enrolling at small number of centers for part A, won't disclose # pts enrolled so far, no drug interactions seen so far. 6/6/11 webcast: still in Part A, hope to progress rapidly to Part B
---Company estimated that it will take about 1 yr to complete (but as of 7/2011 seems like this was way to optimistic).
---Will use 50/50 US and India [80% cheaper and enroll faster because Avastin is not otherwise available] sites. 4/5/11: only US right now.
---4/5/11, 6/6/11 webcasts: Currently doing dose escalation w/ radiation (reduce Azixa by one dose level)- preliminary data suggest no added toxicity. Hope to progress rapidly into comparative portion (so note that Azixa is actually not yet in a randomized p2b portion of the trial). Actively enrolling and hope for preliminary data ASCO 2012.
---2/9/11, 5/10/11, 6/6/11 webcasts: For this trial, have changed from McDonald to RANO progression criteria (published NEJM 2010)- Because it is hard to distinguish between inflammation and progression, keep pt in trial for one further cycle after appearance of possible progression. Better known RECIST criteria is more appropriate for peripheral disease.
---First line advantage- better change of pt response and can get tumor tissue for laboratory studies (2/9/11 and 5/10/11 webcasts: will assess biomarkers in primary tumor specimens and perform additional nonclinical studies- hope to develop a diagnostic to ID biomarkers association with prognosis and response to the drug)
---5/10/11 call: This trial will help design pivotal p3 program and provide supporting data for NDA.
- 5/10/11 call: want to read out data from phase 2b study before deciding whether to partner or develop alone
Azixa program in metastatic melanoma
- Metastatic Melanoma: [62000 diag/yr, 75% of met have brain met, also highly vascularized, stage IV median survival 6-9 months, dacarbazine approved, temo not approved but also used].
- 60-90% of stage 4 melanoma pts have metastases to brain- tends to be the life shortening event (4/5/11 webcast)
- 6/6/11 webcast: Melanoma pts with brain metastases are generally exlcuded from most melanoma trials - because it is assumed/known that the drugs being tested do not cross the BBB
- 2008 initiated open-label phase 1/2 in combo w/ temozolomide (Click here for clinicaltrials.gov link).
---Preliminary data 11/2009 at AACR-NCI-EORTC meeting
---Final data ASCO 6/2010: combo well tolerated [Azixa dose reduction not required, no additive toxicity] median PFS 2.9 months (note phase 3 PFS for TMZ was 1.9 months), In 22 pts, saw 2 PR [4 and 10 months], 9 SD [duration 3-7 months], response rate 50% by modified RECIST criteria.