Navigate the Adolor ADLR Research Pages
- Introduction (valuation, financials, company outlook)
- Entereg (marketed hospital product used to accelerate GI function after bowel surgery)
- ADL5945 (phase 2 product candidate for opioid-induced constipation)
- Pipeline (details on earlier stage assets
2012 Updates
February 2012:
- This product is now in the Cubist $CBST product development pipeline with the new name of CB-5945
- End of phase 2 meeting was held in December 2011 and phase 3 trials will initiate in 2012.
ADL5945 for Opioid-induced Constipation or Bowel Dysfunction (OIC aka OBD) - Intro
- in GI tract, peripheral mu opioid receptors regulate absorption, motility, water secretion - these functions dysregulated when stimulated by morphine, causing OIC/OBD
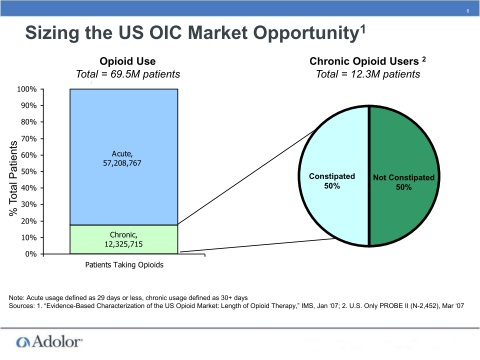
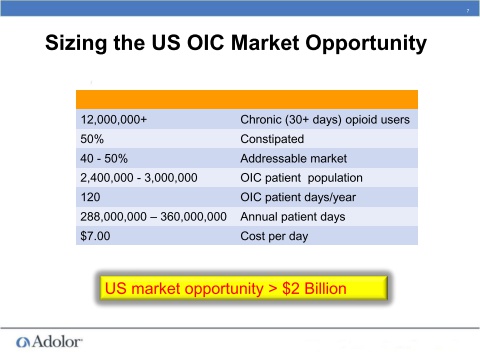
- 230m prescriptions for opioids written in US in 2007, OIC occurs in up to 90% of these patients - significant quality of life and healthcare utilization impact (AZN 4/2011 slide deck).
- Also attempting to identify new drug candidates in this class- but early stage efforts reduced after 7/2010 restructuring
- 2/2011: "millions" of chronic patients taking opioids experience OIC. 3/2011: 270m opioid rx written in 2009, largely generic market but was still growing 4% CAGR
- OIC is a much more common issue with opioid use than dependancy or abuse
- This program targeted for primary care market, so would definitely look to partner "at an appropriate value inflection point"
- ADL5945: (orpa III mu opioid receptor antagonist) in-licensed from LLY 9/2009 for $2m upfront and up to $70m milestones beginning with late-stage clinical trials and undisclosed royalties.
- ADL5945 and ADL7445 COM patents each expire in 2025 (4/6/11 webcast)
- 4/6/11 and 4/28/11: "We are doing everything possible to ensure OIC program is ready for phase 3 on completion of ongoing p2 trials" Goal is to partner "later this year" and start studies in early 2012.
- 4/28/11 webcast: ADLR is engaged in partner discussions now. In the industry there is a good deal of interest in this field (didn't comment on the level of interest in this particular product though...)
ADL5945 - Clinical Trials
- ADL7445: IND filed 9/2009 and phase 1 single dose ascending trial initiated 11/2009 and proceeded to multi-dose 2010
- ADL5945: Phase 1 trials initiated 1/2010 (had to repeat phase 1 studies because changed formulation)
- 2/2011: phase 1 program complete (had healthy volunteers and chronic noncancer pain pts with OIC). Both were safe and well tolerated at therapeutic doses- no severe adverse events reported. ADL7445 designated as backup compound (limited investment in its development contineus as of 3/31/11) and ADL5945 advances. 3/2011: the basis of this decision was discussed at length during Q&A at Cowen but sound was garbled. 4/6/11: ADL7445 is more structurally similar to Entereg. ADL5945 PK is "especially condusive" for this indication
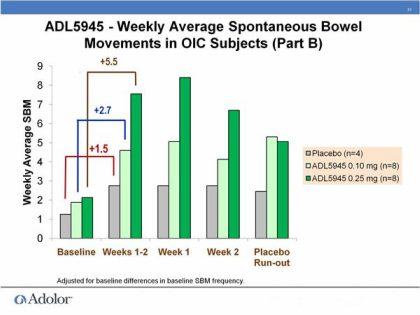
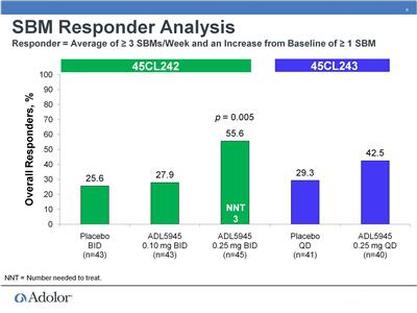
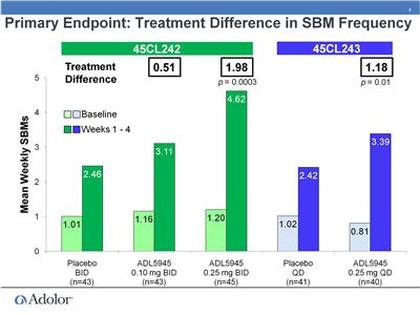
- 3/2011: at Cowen conference, first real discussion of the p1 results (see two slide figures inserted just below). Part A involved cohorts of 1 placebo and 3 ADL5945 patients and determined MTD of 0.25mg BID. Part B demonstrated activity in OIC patients- 4 placebo compared to 8 pts each receiving 0.1 or 0.25mg BID
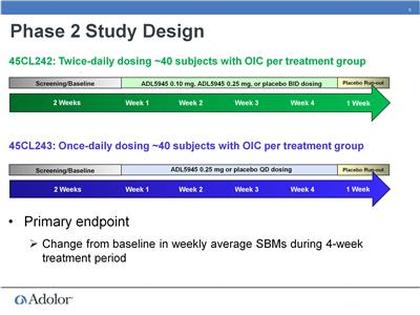
- 10/2010: initiated phase 2 trial of ADL5945 (NCT01207427)- in OIC: placebo, 0.1, and 0.25 mg dosed twice daily, 120 pts, 4 week treatment, primary endpoint= change from baseline in # of spontaneous bowel movements per week
- 1/2011: initiated 2nd phase 2 trial of ADL5945 (NCT01275755) in chronic non-cancer pain: placebo or 0.25 mg once daily, 80 pts, 4 weeks, primary endpoint= change from baseline in # of spontaneous bowel movements per week...to "complete dose assessment in preparation for phase 3"
- 4/6/11 webcast: These trials are using a patient-reported outcomes instrument that is designed to FDA specs
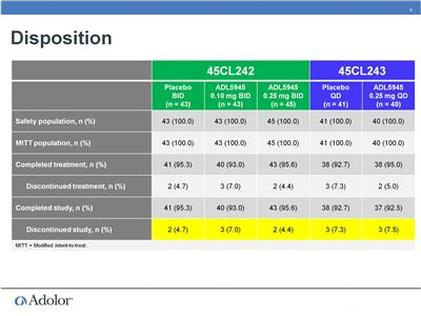
- 6/2011: announced completion of enrollment in both OIC phase 2 trials (click here for PR). Guidance for results in 3q2011 (consistent with guidance at investor conferences in Feb/Mar/April 2011) and phase 3 program to start 1q2012.
- August 2011: announces successful data from phase 2 program (PR) - also see slides below
- 4/6/11 webcast: Would need two pivotal phase 3 12 wk efficacy trials and a 12 month safety study...this would involve "a couple thousand" pts total
Opiod-Induced Constipation (OIC) Program Competition
- Note - I am including drugs in development for the overlapping indication of post-operative ileus (POI) as well, as some drugs are developed for both inidcations (such as Entereg was before safety issues arose with longer term dosing)
- Competitors include drugs from:
- Progenics $PGNX: Relistor is the first approved drug for OIC- click for info (Only approved for patients with advanced late stage disease receiving palliative care). A sNDA has been filed for this subcutaneous drug in less severe chronic pain pts. An oral formulation is also in phase 3 development.
- Tranzyme: Ulimorelin is in p3 for post-operative ileus-click for info and click for p3 initiation announcement
- Helsinn: Ipamorelin is in phase 2b development for post-operative ileus-click for info
- AstraZeneca $AZN and Nektar $NKTR: NKTR-118 is in phase 3 for OIC/OBD-click for info
- Theravance $THRX: TD-5108 is in phase 2 development for GI motility-click for info
- Alkermes $ALKS: ALKS-37 is in phase 2 development for OIC- click for info and click here for Feb 2011 p2 results - 7/2011 update said p2b start 2h2011 with data mid2012 (link)
NKTR-118 for OIC from NKTR and AZN
- NKTR-118 "is a modified (PEGylated) mu-opioid antagonist that inhibits the effect of opioids locally without preventing the CNS analgesic action"
- Licensed by AZN from Nektar 9/2009
- Phase 3 trial enrollment began in 3/2011
- Initial regulatory filings planned for 2013.
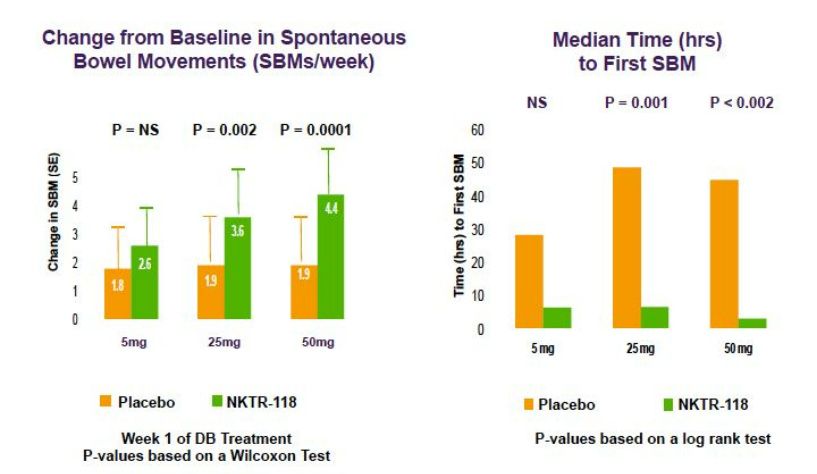
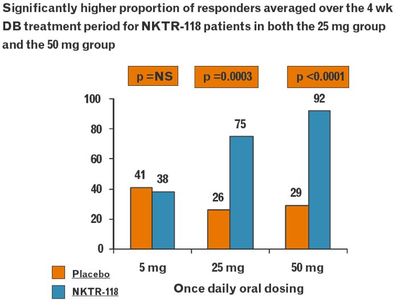
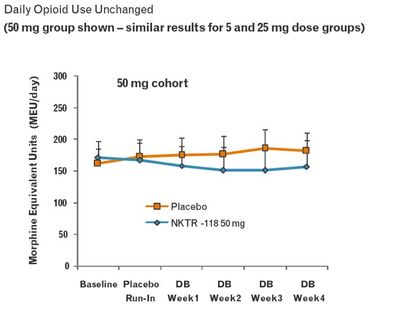
The below figures are from a 2009 NKTR poster (click to download) at an American Association of Pain Management conference