The CELG data, Abraxane's likely FDA approval for pancreatic cancer (NDA anticipate 1h-2013), and the future sales of Abraxane are all key to the value of the CELGZ contingent value right (CVR) issued as part of the Abraxis buyout. Find all the CELGZ financial details here.
Continue reading below for the Celgene abstract as well as a couple of others from Threshold Pharma THLD (phase 2 pancreatic cancer data for TH-302 - not sure if anything new here, please let me know in the comments section if so) and Curis CRIS (another unsuccessful clinical trial of ERIVEDGE/vismodegib) in an indication other than basal cell carcinoma/BCC).
You can search all ASCO GI 2013 abstracts here.
Abstract No: LBA148
Citation: J Clin Oncol 30: 2012 (suppl 34; abstr LBA148)
Author(s):
Daniel D. Von Hoff, Thomas J. Ervin, Francis P. Arena, E. Gabriela Chiorean, Jeffrey R. Infante, Malcolm J. Moore, Thomas E. Seay, Sergei Tjulandin, Wen Wee Ma, Mansoor N. Saleh, Marion Harris, Michele Reni, Ramesh K. Ramanathan, Josep Tabernero, Manuel Hidalgo, Eric Van Cutsem, David Goldstein, Xinyu Wei, Jose Luis Iglesias, Markus Frederic Renschler; Virginia G. Piper Cancer Center at Scottsdale Healthcare/TGen, Scottsdale, AZ; Florida Cancer Specialists, Englewood, FL; Arena Onc Associates, Success, NY; Indiana University Melvin and Bren Simon Cancer Center, Indianapolis, IN; The Sarah Cannon Cancer Center, Nashville, TN; Princess Margaret Hospital and University of Toronto, Toronto, ON, Canada; Atlanta Cancer Care, Atlanta, GA; N. N. Blokhin Cancer Research Center, Russian Academy of Medical Sciences, Moscow, Russia; Roswell Park Cancer Institute, Buffalo, NY; Georgia Cancer Specialists, Georgia, GA; Southern Health, East Bentleigh, Australia; San Raffaele Scientific Institute, Milan, Italy; Vall d'Hebron University Hospital, Barcelona, Spain; START-Madrid, Centro Integral Oncológico Clara Campal, Madrid, Spain; Leuven Cancer Institute (LKI), Leuven, Belgium; Prince of Wales Hospital, Sydney, Australia; Celgene Corp, Summit, NJ; Bionomics Ltd., Thebarton, Australia; Celgene Corporation, Summit, NJ
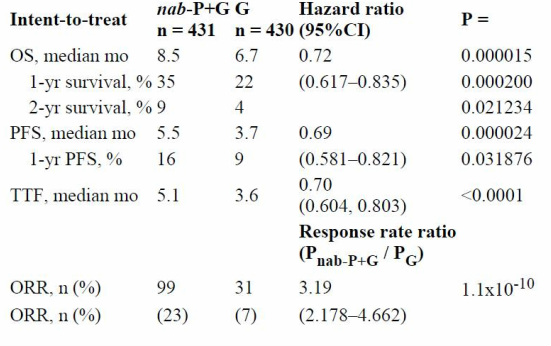
Background: nab-Paclitaxel (nab-P, 130 nm albumin-bound paclitaxel) provides tumor selective localization via transcytosis across the endothelium, potential tumor uptake via macropinocytosis, and improved pharmacokinetics vs cremophor-paclitaxel. In vitro, nab-P increased tumoral gemcitabine (G) levels, and in a phase I/II study in metastatic pancreatic cancer (mPC) nab-P + G showed promising activity.
Methods: Patients (pts) with mPC were randomized to nab-P 125 mg/m2, followed by G 1000 mg/m2 on days 1, 8, and 15 every 4 weeks or G 1000 mg/m2 weekly for 7 weeks (cycle 1), then on days 1, 8, and 15 every 4 weeks (≥ cycle 2). For the primary endpoint of overall survival (OS), 608 events from 842 patients provided a power of 0.9 to detect a HR of 0.769 (2-side α = 0.049). Results: 861 pts received therapy. Baseline pt characteristics were well balanced. Median age was 63 years, Karnofsky performance status was 90-100 in 60% and ≤80 in 40% of pts, 43% had head of pancreas lesions, 84% had liver and 39% had lung metastases, and 52% of pts had CA19-9 ≥59 x ULN. Treatment duration was 4 vs 3 months in nab-P + G vs G. The relative protocol G dose was 75% vs 85% in nab-P + G vs G; nab-P dose was 81%. OS, progression-free survival (PFS), time to treatment failure (TTF), and overall response rate (ORR) were significantly improved in the nab-P + G arm (Table). Most common grade ≥3 AEs were neutropenia (38% vs 27%), fatigue (17% vs 7%), and neuropathy (17% vs 1%) in the nab-P + G vs G arms. Grade ≥3 neuropathy improved to grade ≤1 in 29 days. Febrile neutropenia was reported in 3% (nab-P + G) vs 1% (G) pts.
Conclusions: In this multinational, multiinstitutional study, nab-P + G was well tolerated and superior to G with statistically significant and clinically meaningful results in all endpoints and across subgroups. Clinical trial information: NCT00844649.
Abstract No: 325
Citation: J Clin Oncol 30: 2012 (suppl 34; abstr 325)
Author(s):
David P. Ryan, Shantan G. Reddy, Nathan Bahary, Hope Elizabeth Uronis, Darren Sigal, Allen Lee Cohn, William R. Schelman, Joe Stephenson, Clarence Eng, Mitesh J. Borad; Massachusetts General Hospital, Boston, MA; Louisiana State University Health Sciences Center Shreveport, Shreveport, LA; University of Pittsburgh Medical Center, Pittsburgh, PA; Duke University Medical Center, Durham, NC; Scripps Clinic, San Diego, CA; Rocky Mountain Cancer Center, LLP, Denver, CO; University of Wisconsin Hospital and Clinics, Madison, WI; Institute for Translational Oncology Research, Greenville, SC; Threshold Pharmaceuticals, Redwood City, CA; Mayo Clinic, Scottsdale, AZ
Background: TH-302 is a hypoxia-targeted drug with a hypoxia-triggered 2-nitroimidazole component designed to release the DNA alkylator, bromo-isophosphoramide mustard (Br-IPM), when reduced in severe hypoxia. A randomized Phase 2B study (NCT01144455) was conducted to assess the benefit of G+T to standard dose G as first-line therapy of PAC.
Methods: An open-label multi-center study of two dose levels of TH-302 (240 mg/m2 or 340 mg/m2) in combination with G versus G alone (randomized 1:1:1). G (1,000 mg/m2) and T were administered IV over 30-60 minutes on Days 1, 8 and 15 of a 28-day cycle. Patients on the G could crossover after progression and be randomized to a G+T arm. The primary efficacy endpoint was a comparison of PFS between the combination arms and G alone (80% power to detect 50% improvement in PFS with one-sided alpha of 10%). Overall survival (OS) was a secondary endpoint.
Results: 214 pts were treated; 163 (76%) Stage IV and 51 (24%) Stage IIIB. Median age 65 (range 29-86); 126 M/88 F; 38% ECOG 0/62% ECOG 1. Receiving 6 or more cycles: 32% G; 45% G+T240; 55% G+T340. Median PFS was 3.6 mo in G vs 5.6 mo in G+T240 (p=0.06) and 6.0 mo in G+T340 (p=0.01). Median OS was 6.9 mo in G vs 8.7 in G+T240 (p=0.83) vs 9.2 mo in G+T340 (p=0.80). 6-mo OS was 57% in G vs 69% in G+T240 (p=0.12) and 73% in G+340 (p=0.04); 12-mo OS was 26% in G vs 37% in G+T240 (p=0.18) and 38% in G+340 (p=0.13). RECIST best response was 10% in G vs 17% in G+T240 and 26% in G+T340. 14 and 12 pts in G crossed over to T240 and T340, respectively. Median post crossover PFS was 1.8 mo in T240 vs 2.9 mo in T340 (p=0.13). Median post crossover OS was 2.6 mo in T240 vs 13.4 mo in T340 (p=0.01). AEs leading to discontinuation were: 16% G, 17% G+T240 and 12% G+T340. Rash (47% in G+T340) and stomatitis (42% in G+T340) were greater in combination, 3 pts Grd 3 rash. Grd 3/4 thrombocytopenia were 11% G, 39% G+T240 and 63% G+T340 and Grd 3/4 neutropenia were 31% G, 56% G+T240 and 60% G+T340.
Conclusions: The combination of G plus TH-302 improved the PFS of G. Skin and mucosal toxicity and myelosuppression were the most common TH-302 related AEs with no increase in treatment discontinuation. A phase 3 study of TH-302 (340 mg/m2) in combination with G is planned with OS as the primary efficacy endpoint. Clinical trial information: NCT01144455.
Abstract No: 67
Citation: J Clin Oncol 30: 2012 (suppl 34; abstr 67)
Author(s):
Deirdre Jill Cohen, Paul J. Christos, Joseph A. Sparano, Hedy Lee Kindler, Daniel Virgil Thomas Catenacci, Tanios B. Bekaii-Saab, Sanaa Tahiri, Yelena Yuriy Janjigian, Michael K. Gibson, Emily Chan, Lakshmi Rajdev, Susan Urba, James Lloyd Wade, Peter Kozuch, Erica Love, Katherine Vandris, Naoko Takebe, Howard S. Hochster, New York Cancer Consortium; New York University Cancer Institute, New York, NY; Weill Cornell Medical College, New York, NY; Albert Einstein College of Medicine/Montefiore Medical Center, New York, NY; The University of Chicago Medical Center, Chicago, IL; University of Chicago, Chicago, IL; Ohio State University Hospital, Columbus, OH; The Ohio State University, Columbus, OH; Memorial Sloan-Kettering Cancer Center, New York, NY; University of Pittsburgh Medical Center, Pittsburgh, PA; Vanderbilt-Ingram Cancer Center, Nashville, TN; Montefiore Medical Center, Bronx, NY; University of Michigan Cancer Center, Ann Arbor, MI; Decatur Memorial Hospital Cancer Care Institute, Decatur, IL; Beth Israel Medical Center, Continuum Cancer Center, New York, NY; Investigational Drug Branch, Cancer Therapy Evaluation Program, Rockville, MD; Yale University School of Medicine, New Haven, CT
Background: The HH pathway is overexpressed in gastroesophageal (GE) tumors. Pre-clinically, HH inhibitors have demonstrated a reduction in GE tumor growth, cell motility and invasiveness. V, an oral small-molecule antagonist of the Hh pathway, has previously been safely combined with FOLFOX chemotherapy.
Methods: Pts with untreated, metastatic or locally advanced gastric or GEJ adenocarcinoma were randomized 1:1, stratified by institution and disease status (with or w/o distant mets) to FOLFOX (ox 85 mg/m2, LV 200 mg/m2, 5-FU bolus 400 mg/m2, 5-FU infusion 2400 mg/ m2 over 48 hrs) q14d plus V or placebo (P) (150mg PO daily). Cycle defined as 2 weeks and no crossover allowed at progression. FFPET and blood were collected for biomarker analyses. Response assessed every 8 weeks (RECIST 1.1). Primary endpoint was progression-free survival (PFS), secondary objectives were overall survival (OS), response rate (RR), and toxicity. Results: 124 pts (V/P 60/64) enrolled at 20 sites between 10/09-2/12. Pt characteristics (V/P): median age 58/62; ECOG PS 0: 23 (40%) / 30 (47%); male 39 (65%) / 53 (83%); GEJ 37 (62%) / 39 (61%); diffuse histology 20 (43%) / 14 (30%). Median number of FOLFOX cycles 9.5/11. Most common Gr ≥3 toxicities: (% pts V/P) neutropenia 50.0/31.7 (p=0.07), neuropathy 23.1/14.3 (p=0.33), fatigue 15.4/9.5 (p=0.50), thrombosis 13.5/11.1 (p=0.92), anemia 9.6/9.5 (p=0.99), hypokalemia 9.6/4.8 (p=0.52), nausea 7.7/9.5 (p=0.99). Death on or within 30 days of treatment 6.7%/15.6% (p=0.20). Median PFS in ITT population 11.5/9.3 mo (95% CI 8.5-14.4/6.7-11.9; p=0.34) and median OS 12.2/13.9 mo (95% CI 10.2-14.3/11.5-16.3; p=0.48). Non-statistically significant trends toward improved PFS with V were noted in female pts, diffuse histology, and PS 1 (p≤ 0.08). RR (%) 33/27 (p=0.64).
Conclusions: Addition of V to FOLFOX did not improve PFS in an unselected advanced GE carcinoma population. Blood and tissue biomarker analyses are ongoing to determine if there is a subset of patients who may derive benefit from V. Supported by: N01-CM-62204, -62201, -62207, -62206, -62209, -62208 and 2UL1 TR000457-06 Clinical trial information: NCT00982592.
 RSS Feed
RSS Feed
