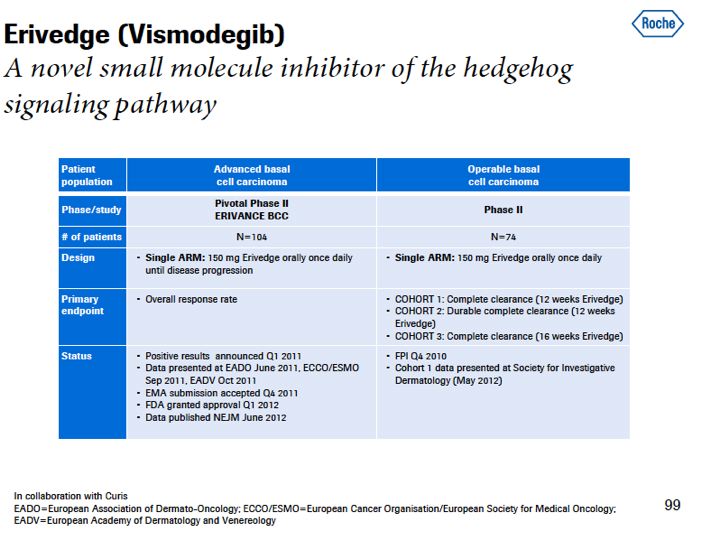
"On Erivedge, the rollout is beginning in the U.S. We're expecting the approval sometime in 2013, a very powerful product for patients with basal cell carcinoma. We are still learning our way through this product, I would say, because these patients are not always treated by oncologists, sometimes they're treated by dermatologists. And trying to find these patients in the network is something that we're learning, to a certain extent, as we go. But the efficacy of the treatment is helping us to guide us as we progress through that in the U.S. And of course, there are different prescribing patterns as well in Europe that we're preparing ourselves for, for that launch."
From press release:
Sales of Erivedge, which is used to treat advanced basal cell carcinoma, were 29 million Swiss francs [~$32m USD]. Sales volume is on track since its US launch in February [2012].
The slide below is from Roche's most recent investor/analyst day presentation.
"Erivedge launch: first in class, first to patients
At 8:00 am on Monday, January 30, 2012, Roche received FDA approval for Erivedge, setting off a remarkable coordinated effort by the Roche team and our contract manufacturers. With the active pharmaceutical ingredient sourced in Asia and Switzerland and the capsule produced in Canada, what remained was packaging and shipment. The Roche artwork experts together with a printing company worked around the clock to complete the packaging design, based on the final wording of FDA approval, and prepare the actual packaging. With Roche staff on site at 3:00 am on Tuesday to oversee packaging, the initial two lots were loaded that afternoon on separate trucks to mitigate against damage or delay. By Wednesday morning, the new treatment was safely in our warehousing and distribution centre in Louisville, Kentucky (US). And when the FDA cleared it for shipment on Thursday, Erivedge was on its way to the distributors within a few hours."
 RSS Feed
RSS Feed
