LX4211
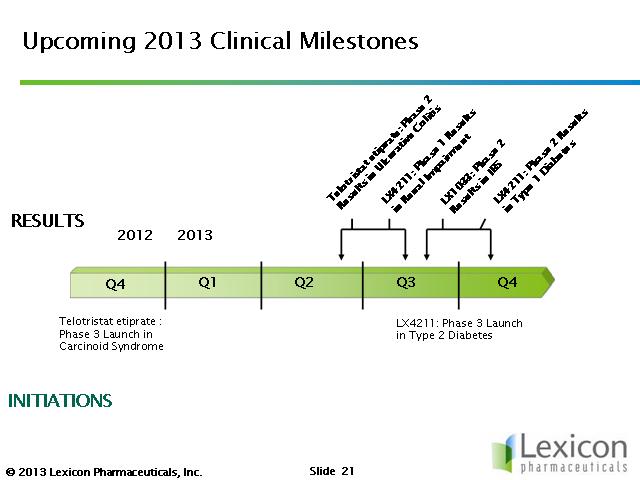
- there is a study ongoing in 20 paientts with moderate renal impairment - very common in T2D and they have few treatment options. 400 mg vs placebo, results 1h2013.
- we are initiating a 28-day study in type-1 diabetes (T1D), 33 pts in combination with insulin vs placebo. looking to reduce total insulin use plus measures of glycemic control, now in enrollment phase
- "We’re also finalizing our late-stage development and commercialization strategy...We’ve completed our discussions with the FDA and EMA and we feel that our design for the Phase 3 program is robust, and we’ll get labeling for treatment of a broad diabetic population. The program will evaluate LX4211 alone and in combination with other agents. It will include studies in the renal impairment, and our late-stage development will include work in type 1 diabetes. We will also continue with our plans to show mechanistically synergy with DPP-4 Inhibitors, and that will give us some insight into the potential of combining LX4211 with a DPP-4 inhibitor in actual practice. These leverage the unique dual mechanism of action with the SGLT1 and SGLT2 inhibition of LX4211. And the program does include a large cardiovascular outcome study because we’re focusing as well on the potential to demonstrate cardiovascular benefit given the glucose reduction, weight reduction and blood pressure reduction. We feel we’re well on track to initiate Phase 3 with a corporate partner in 2013."
- company won't disclose # of studies or patients in the phase 3 program at this time
- See more below re financials and partnering
- My summary the Q&A session:
-Partnering - we are talking to multiple parties. Looking for a deal that will fund the program, but we want to have significant involvement.
-Cardiovascular outcomes trial to be completed at time of filing...don't those, um, take forever? Similar strategy as canagliflozin- start the trial during phase 3 and have an interim data analysis included in the NDA..."But the complete set of data to provide the most robust results comes in really around the time of approval, or certainly after approval, in order to have even more follow-up and more definitive cardiovascular data"
LX2761
- Inhibitor of SGLT1 only
- In IND-enabling toxicology study now, expect phase 1 early 2014
- Drug stays in GI tract, virtually no systemic exposure
- No comment on whether this would be included in LX4211 partnership, would vary depending on discussions
Telotristat (LX-1032)
- phase 3 ongoing in carcinoid syndrome. A single pivotal trial with 12 weeks of placebo control. 250 or 500 mg 3x daily, target 105 patients, hope to demonstate reduction in bowel movement frequency. Goal to complete enrollment in 2q2014, results in q3/q4 2014 - this is an aggressve goal.
- "We’re in the process of getting sites up and running. We’ve already randomized as well. We’ve had our investigator meetings in the U.S. and in Europe, and most recently in Australia. So, we’re in the process of really getting a lot of sites on board and we’ll look forward to ramping up the study in 2013."
- phase 2 proof of concept (POC) in IBD continues..."assessment of the relationship between serotonin and efficacy in ulcerative colitis" 60 mild to moderate UC patientts, 500 mg once daily (qD) or 3x daily, for 8 weeks with mesalamine. Expect to complete enrollment in 2q2013.
LX1033
- phase 2 ongoing for inflammatory bowel disease (IBD) - looking at changes in stool consistency
- "It was designed as a result of the FDA meeting in 2011, in order to solidify the elements of our patient reported outcome strategy and to find the specifics of our biomarker plan. Enrollment is ongoing where more than two-thirds of the way through. We anticipate our trial completion in the third quarter of 2013."
- "I think we’re very pleased, obviously, with our proof-of-concept data with LX1031, the predecessor compound, and particularly when we reanalyzed the data, the published data, with new FDA guidelines for both entry criteria and the dual endpoint of pain and stool consistency improvement. And when we reanalyzed using the new FDA entry criteria and dual efficacy endpoint, we had a 52% response on our active and about 14% on placebo. I think that bodes well for anywhere in the ballpark of that kind of benefit relative to placebo. It would be a tremendous success and we’d be moving forward to Phase 3 aggressively."
LX2931
- Proof of concept data in 2012, currently considering next steps, if any.
- Positive results in rheumatoid arthritis in small study - consider moving forward in RA or different indication
- "So we’re brainstorming on potential alternative indications that might actually make more sense for this mechanism. And the competitive landscape of RA has changed, and we may find a more advantageous avenue for that in the area of autoimmune disease. So there’s still is a path – a potential path forward, but it’s one that requires, I think, some recalculation."
LX7011
- Proof of concept data in 2012 - positive results in lowering of intraocular pressure for glaucoma
- Requires reformulation to improve efficacy, making plans for that, which would include additional preclinical testing
- Both 2931 and 7011 described as "on hiatus" right now
Corporate Guidance
- $223m cash at year-end 2012
- 512 million shares outstanding, plus 21.5m options
- 2013 expenses of $110-120m with cash use of $92-97 million.
- "We are engaged in partnership discussions for LX4211 as you know, and are also in conversations about other potential collaborations and alliances...I should note that these operating expense and net cash use expectations reflect cost of preparations that we are making for Phase 3 development of LX4211, as well as certain supportive non-clinical and clinical activities. But they did not reflect the total cost of full scale Phase 3 clinical trials for that program given our expectation of a partnership around those activities."
 RSS Feed
RSS Feed
