"The second potentially transformative candidate in our pipeline is our BACE inhibitor for the treatment of Alzheimer's disease. BACE has been a very difficult target for the entire pharmaceutical industry and our chemists have made incredible progress. Today we are delighted to show you exciting data demonstrating an unprecedented reduction of CSFA data levels in humans with MK8931, our lead BACE inhibitor which is in Phase I of development
Here's one example of where modeling assimilation has enhanced our decision making.You will hear more about our base inhibitor program for Alzheimer's disease later from Darryle Schoepp.When we initially took our lead BACE inhibitor into humans, we found that the molecule was much more efficacious than we had anticipated. As a result, at all doses studied,
we observed very robust lowering of CSFA beta line.This is illustrated in the boxed area on the graph. Traditionally, to ensure that we would have a good dose response in our Phase II study, we would have needed to conduct an additional Phase I study at lower doses before selecting doses to manufacturer for Phase II. However our modeling and simulation experts were able to create mechanistic models to predict the CSFA beta response at lower doses. This allowed us to select doses for Phase II drug supply manufacturing without delay while we conducted a confirmatory Phase I study in parallel, enabling an early Phase II start."
----
"So I will end by giving you an update in an important and exciting Merck program, BACE inhibitors for Alzheimer's disease progression. Alzheimer's disease is an irreversible neurodegenerative brain disease that leads to dementia with cognitive behavioral changes
that are associated with functional impairments of activities of daily living and ultimately death.Today, there are no treatments available to stop of slow disease progression over the long term or to prevent it. Brains of Alzheimer's patients are characterized by amyloid plaques, neurofibulatory tangles, inflammation and neuronal death. About 35 million people live with dementia around the world. In the United States there are over 5 million patients with
Alzheimer's disease. About one out of every eight persons, 65 years of age or older, suffers from Alzheimer's diseases.The costs to payers are staggering, estimated to be $183 billion in the US during 2011. Worse, the prevalence of Alzheimer's disease is growing rapidly, due to the aging population, creating an unsustainable burden on patients, caregivers and the economy. If no effective treatments are found by 2050, its estimated there will be over 13 million
Alzheimer patients in the United States, payer costs will grow to over $1 trillion. These statistics do not begin to capture the emotional and physical toll Alzheimer's disease takes on patients and their families.
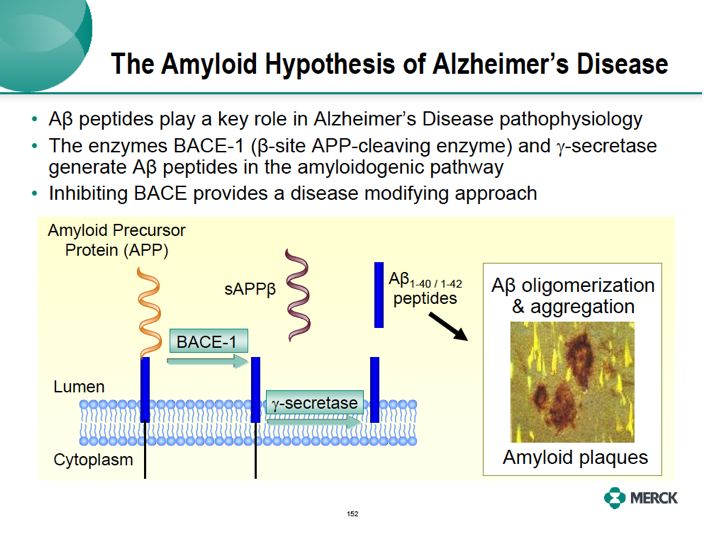
The amyloid hypothesis is a leading therapy of what causes Alzheimer's disease. A positive role of amyloid is supported by genetic studies in patients to familiar Alzheimer's disease who get early onset disease.These patients have gene mutation that lead to overproduction of amyloidogenic A beta peptide and amyloid plaques.The presence of amyloid plaques was originally observed at autopsy in the brains of Alzheimer patients over 100 years ago.
Now been show are people at high risk for Alzheimer dementia using brain imaging with amyloid PET labeling. The two key enzymes that generate A beta peptide and the amyloidogenic pathway, beta secretase (BACE) and gamma secretase. These enzymes process amyloid precursor protein, APP to produce short peptide forms A beta 1-40 and 1-42, which then aggregate and produce amyloid plaques. Amyloid is associated with loss of neurons, brain atrophy and ultimately clinical symptoms. Inhibitors of gamma secretase have been tested in Alzheimer disease trials, but cannot be administered at doses which substantially reduce A beta peptides in the human brain.The enzyme gamma secretase also processes other substrates, in particular Notch A protein that's needed for normal function of tissues throughout the body. This has limited doses of these compounds in the clinic only to those which have very modest effects on formation of A beta peptide in the brain. Merck has been working on BACE -- BACE inhibitors as a way to more robustly inhibit the amyloidogenic pathway while potentially avoiding the safety and tolerability concerns of the gamma secretase inhibitors.
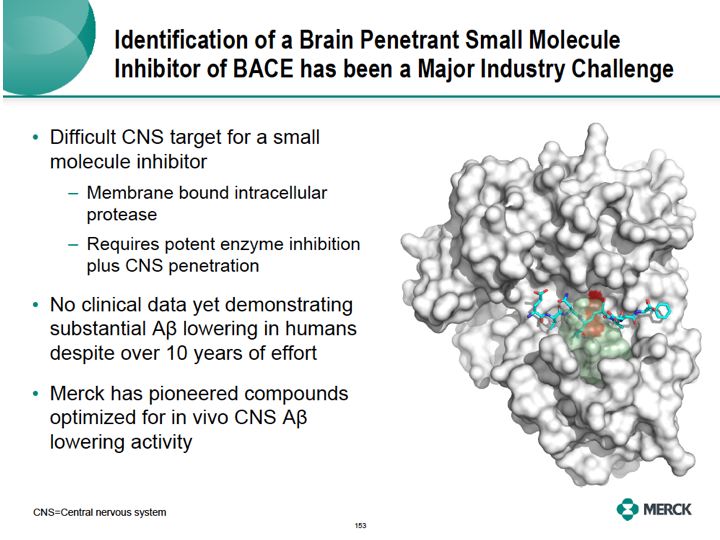
Discovery and identification of a small molecule BACE inhibitor that readily enters the brain to reduce amyloid has been a major challenge. This has been a difficult target for several reasons. BACE is an aspartic protease enzyme compartmentalized to the inner membranes of neurons.
Its active site is relatively large in order to provide access to the proteins of processes. Such as amyloid precursor protein, or APP. The active side of the enzyme is illustrated on the right, showing a peptide substrate docking to a shallow active site. A small molecule must not only [?? word] the active site to inhibit the enzyme, but must enter into the CNS and inside of the
neuron with sufficient concentration. Despite over 10 years of intense effort to discover such molecules, there's still no BACE inhibitor in clinical development with substantial [senis] A beta lowering in humans. Merck who's been pioneering the design of brain penetrate molecules optimized for in vivo senis A beta lowering activities. The orange and the red regions are the enzyme active site in the figure represents critical regions where small molecule inhibitors bind. Unique insights from the use of structural modeling by our chemists were keyed in the success of our program.
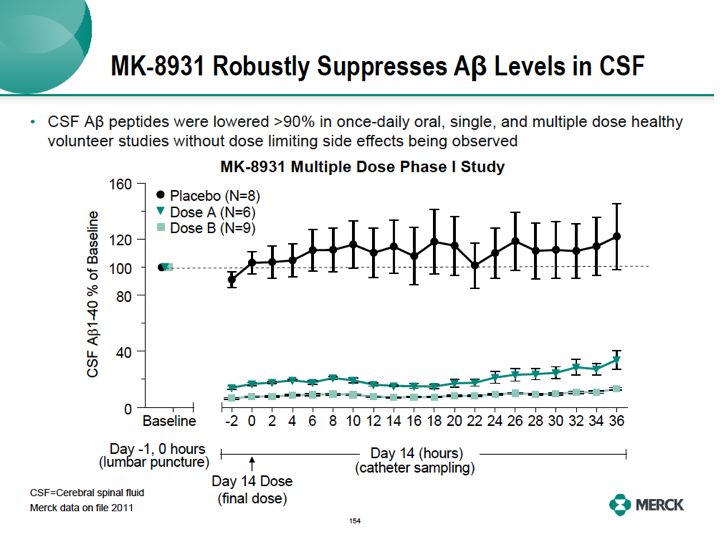
As a result of these efforts, extremely pleased to now show you clinical data with our lead BACE inhibitor molecule. Our lead Merck BACE inhibitor, known as MK8931, greatly suppressed A beta peptide levels in the human brain.These are the results of our multiple dose Phase I study with MK8931.These studies are intended to find doses that reduce A beta peptides in the brain, using the cerebral spinal fluid, that's CSF compartment and examine the safety and tolerability of these doses in humans. Volunteers were given the compound or placebo orally once per day for 14 consecutive days. A baseline value of A beta peptide includes SAPP beta, A beta 1-4, MA beta 1-42 was determined prior to the first dose by lumbar puncture of the CSF. Subsequent data is calculated as a percentage of that value. On day 13 of dosing, a few hours prior to dose 14, the last dose, the catheter was inserted in the lumbar space with serial CSF samples. Last dose is given and CSF samples were collected every two hours for 36 hours.When compared to placebo, both a lower dose A and a higher dose B of our compound markedly inhibited the formation of A beta 1-40 in the CSF were up to 36 hours past the last dose. Very similar reductions, not shown here, were also observed for SAPP beta and A beta 1-42 peptide. This study shows the Merck base inhibitor, MK8931 reduced CSF A beta peptide by greater than 90% in healthy volunteers without observing dose limiting side effects in this study. Details of these Phase I studies will be presented at scientific meetings in 2012.
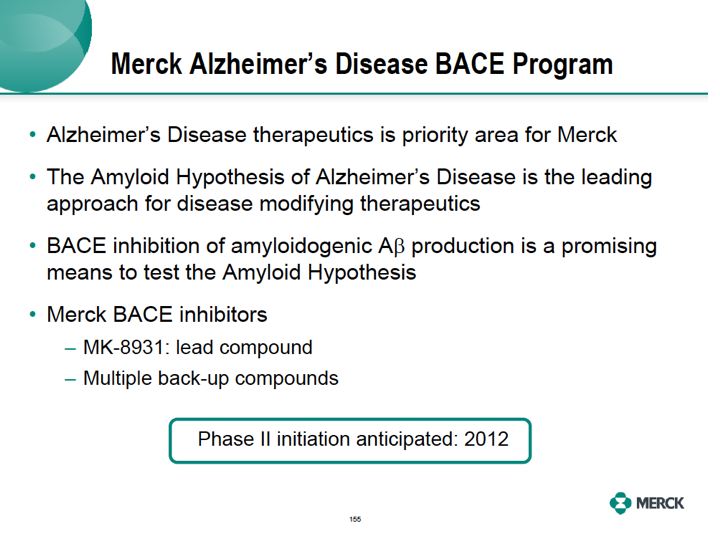
Alzheimer's disease therapeutics, the priority area for Merck, the amyloid hypothesis remains a leading approach for disease modification for Alzheimer's disease. Based on our study BACE inhibition is a very promising means to inhibit amyloidogenic A beta production with a goal of preventing or delaying Alzheimer's disease progression. Phase one studies with our lead first
BACE inhibitor, MK8931, showed potent lower of A beta peptides in the CNS and it was generally well tolerated in those studies. We also have multiple back up compounds and I'm pleased to announce that we expect to initiate our Phase II studies in patients
with Alzheimer's disease in 2012."
From Q&A session:
"And then last question is on Alzheimer's disease. I know you guys have kind of behind the scenes been doing mechanistic work for a number of years on Alzheimer's and the compound you highlighted is a BACE inhibitor which is small molecule and can go into neurons for example and target A beta. Can you speak in your opinion about the validity of the approach of targeting
A beta accumulation outside of the neuron, and where do you think that's a valid approach? Because that's essentially what the monoclonals do?
I don't really think that we want to comment on the other methods for removing A beta, in particular the antibodies that are being used to remove A beta. As you know, we'll see some of the results of the trials that are ongoing in the next few years and it'll be interesting to see what happens. I will say that we're just extremely excited about the degree of inhibition that we're getting with BACE.We've never seen anything like it and like I said, in my remarks, we grossly overshot it when we first did the Phase I experiments, because we just never expected to see that. And as Darryle said, we're able to get greater than 90% inhibition of CSFA beta, SAPP as well as 1 to 40 and 1 to 42 with our drug, without having a dose limiting toxicity.
And so we think that this is the molecule that will definitively provide the best test of the A beta hypothesis for Alzheimer's disease as we move forward and as Darryle mentioned and I also mentioned, when you just look at what the chemists had to do to get this, it is an unbelievably impressive story and it really just a complete chemical structural biological tour de force to
get this molecule to the point where it is and it's something that we're really excited about and as Darryle said, we have backups in the series that also are showing very nice efficacy"

 RSS Feed
RSS Feed
