- The American Association for the Study of Liver Diseeases (AASLD) meeting abstracts are now available - this is a key scientific conference for Hepatitis C virus (HCV) drug developers
- Ligand $LGND partner GSK will present data from the first of two phase 3 trials for Promacta in HCV at this meeting. The full text of the abstract and associated figure is below.
- Find more info on LGND here and a complete run-down of upcoming biotech events here.
N. Afdhal1; G. Dusheiko2; E. G. Giannini3; P. Chen4; K. Han5; A. Moshin6; M. Rodriguez-Torres7; S. Rugina8; E. Lawitz9; M. L. Shiffman10; G. Tayyab11; F. Poordad12; Y. Mostafa Kamel13; A. Brainsky14; J. Geib14; S. Y. Vasey14; R. Patwardhan14; F. M. Campbell13; D. Theodore15
Purpose: Thrombocytopenia, a common complication of cirrhosis due to hepatitis C virus (HCV) infection, limits the ability to initiate and maintain antiviral therapy (peginterferon [PEG] alpha+ribavirin [RBV]). ENABLE 1 evaluated the ability of eltrombopag to increase platelets, allow initiation and maintenance of antiviral therapy, and increase sustained virological response (SVR).
Methods: Chronic HCV patients (pts) with baseline platelets <75,000/µL received open-label oral eltrombopag (25mg daily w/ escalations every 2 wks to 100mg) until platelets were ≥90,000/µL (Part 1). Pts achieving these counts were randomized 2:1 to eltrombopag or placebo (Part 2) in conjunction with PEG alpha-2a (40KD; 180µg/wk) + RBV (genotype 2/3: 800mg daily; non-genotype 2/3: 1200mg [1000mg if <75kg] daily). Pts were treated for 24 wks (genotype 2/3) or 48 wks (non-genotype 2/3). The primary endpoint was SVR. Stratification variables were baseline genotype (2/3 vs non-2/3), baseline platelets (<50,000 vs ≥50,000/µL), and HCV RNA at screening (<800,000 vs ≥800,000 IU/mL). Efficacy analyses were performed on an intention-to-treat basis.
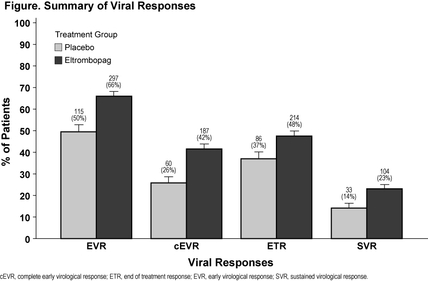
Results: 715 pts, 78% with bridging fibrosis or cirrhosis (FibroSURE™ score equivalent to METAVIR F3-F4), entered Part 1. Median baseline platelets were 59,000/µL and increased to 89,000/µL by wk 2. 682 pts (95%) were randomized to HCV therapy (eltrombopag, 450; placebo, 232). Baseline characteristics were similar between arms. Median platelets at randomization were 133,000/µL and 128,000/µL for the eltrombopag and placebo arms, respectively, decreasing to 90,000/µL and 43,500/µL by wk 4. Throughout Part 2, median platelets were >80,000/µL and <50,000/µL for the eltrombopag and placebo arms, respectively. Eltrombopag was associated with a longer interval to first antiviral dose reduction (hazard ratio=0.41; stratified log-rank P value<0.0001) and a lower proportion of antiviral dose reductions (Wilcoxon rank sum P value=0.0029). Higher rates of virological responses were observed with eltrombopag (Figure): 23% of eltrombopag pts achieved SVR vs 14% of placebo pts (P=0.0064). The overall adverse event profile was similar between treatment groups, including thromboembolic events. More pts on eltrombopag vs placebo had mild abnormalities in renal parameters; most resolved without interruption of eltrombopag.
Conclusion: Treatment with eltrombopag increased platelets in thrombocytopenic HCV-infected pts with advanced fibrosis and cirrhosis, enabling 95% to initiate antiviral therapy. Compared to placebo, eltrombopag allowed significantly more pts to maintain antiviral dose and obtain SVR.
 RSS Feed
RSS Feed
