Simply put, if AMR101 is designated as a New Chemical Entity (NCE) by the FDA, the product receives five years of market exclusivity after approval in the United States, regardless of the patent situation (sometimes referred to as Hatch-Waxman exclusivity). During this time the FDA will also not accept or approve ANDA's or 505(b)(2) applications for generic versions (except in case of ANDA with Paragraph IV certification that can be filed 1 year before NCE exclusivity expires).
It is important to note that a product can receive a 3 year period of market "data exclusivity" if it contains an active moiety (see definition below) that has been previously approved but has been the subject of new clinical investigation by the sponsor that were essential for approval. FDA will not approve any ANDA's during this time, but they can be filed. Amarin's efforts with AMR101 clearly meet this threshold, so I will not discuss this point further.
Keep reading below the jump for the rest of my discussion and analysis:
Here is the definition according to FDA code: "New chemical entity means a drug that contains no active moiety that has been approved** by FDA in any other application submitted under section 505(b) of the act."
** Note that approved refers to the active ingredient alone or in combination (see #6 at this link)
In turn, the definition of active moiety is "the molecule or ion, excluding those appended portions of the molecule that cause the drug to be an ester, salt (including a salt with hydrogen or coordination bonds), or other noncovalent derivative (such as a complex, chelate, or clathrate) of the molecule, responsible for the physiological or pharmacological action of the drug substance."
Are New Molecular Entity (NME) and NCE synonymns?
While they are very similar the definitions of NCE and NME are not identical. A new molecular entity is "a drug that contains an active moiety that has never been approved by FDA or marketed in the US"
Importantly, it is NCE status, and not NME status that governs the granting of the five year exclusivity period.
When is NCE status granted?
NCE status is granted upon approval of the New Drug Application (NDA). AMRN submitted its first NDA for AMR101 on or around 9/26/2011, so if the FDA accepts the filing for review, approval and potential NCE status could come in late March 2012 (if Priority Review) or late July 2012 (if Standard Review).
Vyvanse
Another example of a NCE controversy that I have seen a few people bring up is Vyvanse, an ADHD drug from Shire. This drug (lisdexamfetamine dimesylate) is a prodrug that is metabolically converted to dextroamphetamine (aka Adderall, a previously approved drug). The FDA approved the drug and granted NCE status and 5 years market exclusivity. Generic drug maker Actavis sued the FDA over this decision. The FDA reviewed the case internally and reaffirmed its decision making, and also won the lawsuit against Actavis. In my opinion, this situation has essentially no applicability to the AMRN case.
FDA Law Blog (3/2009) - Actavis sues FDA.
Pharmawire (10/2009) - FDA likely to prevail in lawsuit and uphold NCE.
Shire PR (10/2009) - FDA determines that it properly granted NCE status.
EMEND
This is a second example of a prodrug gaining NCE status (in this case the FDA reversed its original decision and later decided to give the 5 year exclusivity)
FDA Law Blog (1/2010) - FDA reverses course and grants NCE.
EMEND (aprepitant capsules; original molecule approved) detailed product info.
EMEND (fosaprepitant dimeglumine prodrug for injection) detailed product info.
Vermyst
Another controversial NCE case is Vermyst (fluticasone furoate), which is an ester of FLONASE (fluticasone propionate). The Veramyst product does contain the fluticasone propionate molecule, so it could have been determined that Veramyst would not qualify for NCE status. However, GSK successfully argues that "the furoate group remains an integral part of this new chemical entity while exerting therapeutic activity at the site of action, and reviewers should appreciate that neither fluticasone furoate nor fluticasone pripionate is ever metabolized to fluticasone"
FDA Law Blog (7/2009) - The Stable Ester and NCE Exclusivity.
Correspondence between GSK and the FDA on this matter.
Pancreatic Enzyme Products
These products (current brands include Cotazym, Creon, Pancreaze, and Zenpep), consisting of active ingredients amylase, lipase, and protease (collectively pancrelipase) are purified from mammalian sources. Similar products had been on the market predating the Food Drug and Cosmetic Act of 1938. In 2004 the FDA declared that companies wanting to continue marketing the products would have to submit NDAs. Despite products with the same active ingredients being marketed previously, the FDA determined that these complex mixture products could not be sufficiently characterized to determine if the active ingredients from different companies were identical. Therefore, the FDA decided to give each manufacturer the benefit of the doubt by considering their drugs to each be NCEs and each received 5 years of exclusivity.
Heparin-derived Products
"Unfractionated" heparin is derived from the intestine of pigs and has anticoagulation properties. According to the FDA, the active ingredient in these products is "heparin sodium." Today, there are many improved products on the market consisting of so-called low molecular weight heparins (LMWHs) that are produced by further refining and processing heparin to improve safety and efficacy. One such example is Lovenox (active ingredient enoxaparin sodium, a frequent topic of discussion on this blog). While heparin has fragments ranging from 3-30 kDa in mass (average 12-15 kDa), Lovenox has a much smaller average molecular weight of ~4.5 kDa. Lovenox was approved in 1993 and was granted NCE status. Compared to the above examples, the LMWHs are much more applicable to AMR101, and in my opinion offer support for granting NCE status to AMRN - see further discussion below.
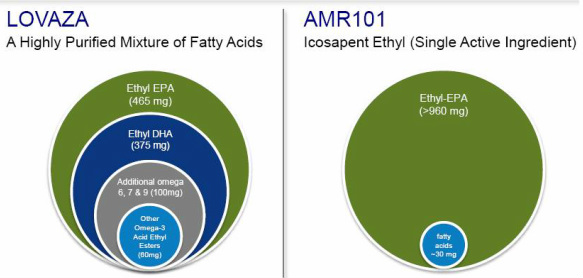
Lovaza
A significant factor in determining whether AMR101 qualifies for NCE status is the comparison to Lovaza, an omega-3 fatty acid product derived from fish oil that is sold by GlaxoSmithKline $GSK as a drug to lower triglycerides. Per the FDA's drug database, the active ingredient is "Omega-3-Acid Ethyl Esters." We can find additional details on the FDA product label: Lovaza consists of a 1 gram capsule containing at least 900 mg of the active ingredient sourced from fish oils. Each capsule contains ~465 mg of the ethyl ester of eicosapentaenoic acid (EPA) and ~375 mg of docosahexaenoic acid (DHA).
So two key questions would seem to be:
1) Is "ethyl-EPA" a new active ingredient?
2) Does AMR101 contain "Ethyl Omega-3 Acids," the active moiety in Lovaza?
When I set out to research this topic, my first impressions were that the answers to these questions were:
1) Yes. I think the "active ingredient" for AMR101 when approved will be listed as "Ethyl-EPA" instead of the "Ethyl Omega-3 Acids."
2) Yes, because ethyl-EPA would seem to be included in the broader class of "Ethyl Omega-3 Acids."
In this case, AMR101 would be considered a New Molecular Entity, but would not qualify for New Chemical Entity status and the 5 years of market exclusivity.
However, here I think the case of the LMWH products such as Lovenox offer some insight. Using the same logic, no LMWH product could be a NCE, because Lovenox and the other new drugs would all contain the previously-appoved "heparin." In both cases, additional scientific study and manufacturing/purification processes have been brought to bear upon the more raw drug (fish oil in Lovaza or unfractionated heparin), generating new, not yet approved, active moieties.
The bottom line? I'll stand by my prior tweet "anyone who acts like it [NCE status] is slam dunk for $AMRN is misguided." However, I now feel like the odds of Amarin gaining NCE status for AMR101 is better than even. I guess we will have to wait and see until sometime next year!
Thanks to many on Twitter and the Investors Hub Biotech Values board for stimulating and helpful discussions that assisted in my research for this article. Please feel free to continue the discussion by leaving a comment here or emailing me.
 RSS Feed
RSS Feed
