Navigate the OncoGenex OGXI research pages
Introduction (Financials, pipeline, milestones, catalysts, general info and links)
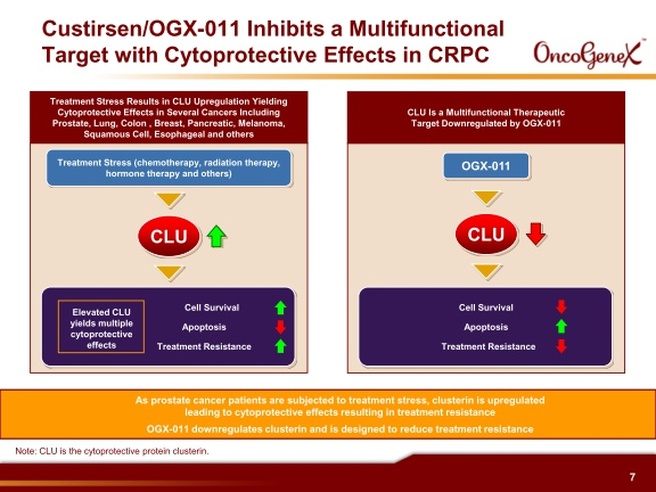
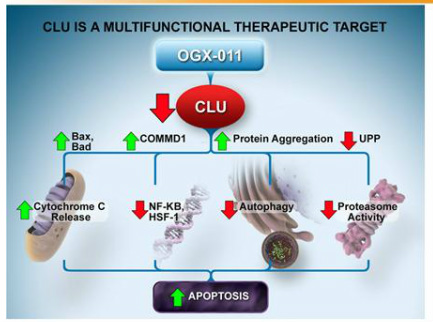
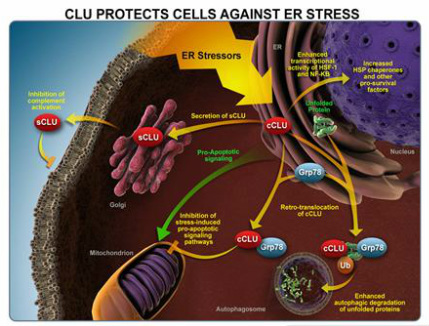
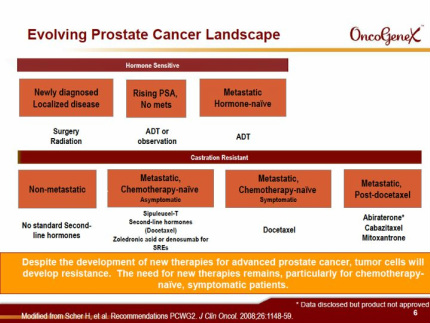
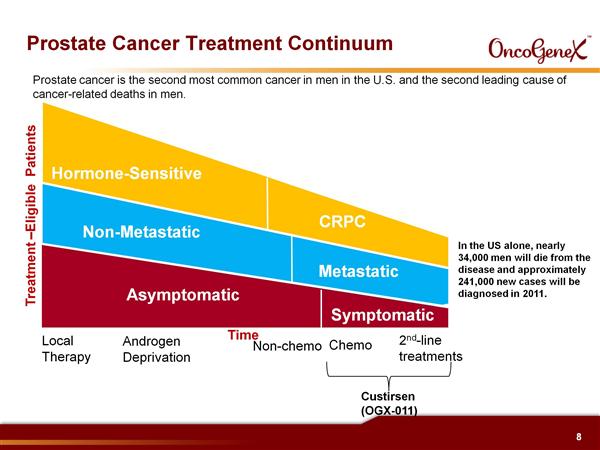
Custirsen aka OGX-011 (phase 3 asset for prostate and lung cancer partnered with TEVA, antisense targeting clusterin)
OGX-427 (phase 2 prostate and bladder cancer asset, unpartnered, antisense targeting HSP27)
Early pipeline products (all preclinical and focused on addressing mechanisms of cancer treatment resistance)
Custirsen aka OGX-011 (phase 3 asset for prostate and lung cancer partnered with TEVA, antisense targeting clusterin)
OGX-427 (phase 2 prostate and bladder cancer asset, unpartnered, antisense targeting HSP27)
Early pipeline products (all preclinical and focused on addressing mechanisms of cancer treatment resistance)
Custirsen (aka OGX-011 aka TV-1011) Intro
- 2nd generation antisense drug against clusterin
- Partnered w/ Teva worldwide 12/2009 for $50m upfront and up to $370m milestones, 15-25% royalties, $10m investment at $37.38 - 20% premium, OGXI must fund $30m out of $170m estimated trial expenses, predicted to meet this 4q2012, $9.0m so far as of 3/31/11, this amount includes some OGXI personnel costs and does not change if decide to pursue any other indications- Teva would pay for those in full), OGXI retains option for US/Can co-promote.
- In 11/2001, OGXI licensed the drug from Isis Pharma (click here for research page), which is entitled to 30% of milestones and royalties of 6.4-7% (thru 2017, then decrease to 3.9-4.5% due to end of royalties owed by Isis to 3rd party). Originally was a 65/35 split, but amended in 7/2008 to give full responsibility to OGXI. 2010 10k: do not expect any payments to Isis in 2011. Companies differ as to whether Isis is owed 30% of sales-based milestone payments. A $10m milestone is owed to Isis on change in control (any milestones between now and then would decrease this), which also would trigger a switch to the maximum royalties due to Isis (this royalty rate change does not apply if the acquirer is Teva). If OGXI does not spend the $30m by 12/2012, ISIS is owed 30% of the unspent amount less $3.5m.
- Licensed IP related to clusterin from U. British Columbia, owe small milestones ($0.3m paid on Teva deal signing and 1% of nonroyalty milestones) and royalties. Patent expires US 2021 and ROW 2020.
- Granted US use patent in 2009 that expires in 2021
- Received FDA fast-track status for CRPC in combination with 1st or 2nd line docetaxel
Custirsen Clinical Trials
- Both phase 3 prostate cancer trials have FDA SPA's and EMEA written scientific advice agreements
- 6/2010 initiated p3 SATURN trial in 2nd-line CRPC of combination docetaxel retreatment/prednisone +/- custirsen (Click for clinicaltrials.gov link, 300 pts, 50 sites- 20-25% EU sites, 12 wk pain palliation as primary endpt. This trial conducted by by OGXI, continue treatment until pain progression/toxicity). Eur sites 3q2010). WIll not be enrolled by ye2011 (4/2011 at Needham)
- 1q2011: OGXI submitted proposed protocol amendment to expand the inclusion criteria and allow either docetaxel retreatment OR cabazitaxel as the 2nd line chemotherapy agent. Randomization remains to custirsen or placebo, then stratify based on chemotherapy chosen. The two chemo agents showed similar pain palliation in their trials so does not affect statistics- have assumed 10% palliation in control arm. Goal is to remain aligned with current treatment paradigms
- 9/2011 announced successful SPA amendment (PR).
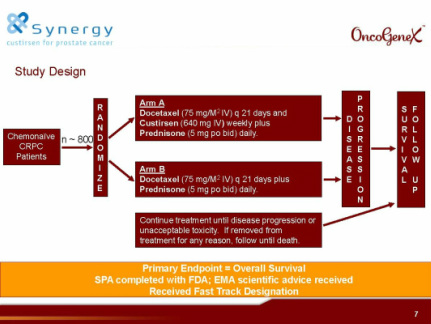
- 9/2010: initiated 2nd p3 trial SYNERGY in 1st-line CRPC of combination docetaxel +/- custirsen (Click for clinicaltrials.gov link, 800 pts, OS as primary endpt. this trial conducted by TEVA, higher % intl sites compared to SATURN-125 total worldwide [123 as of 1q11 results], continue treatment til progression/toxicity). Will not be enrolled by ye2011 (4/2011 at Needham)
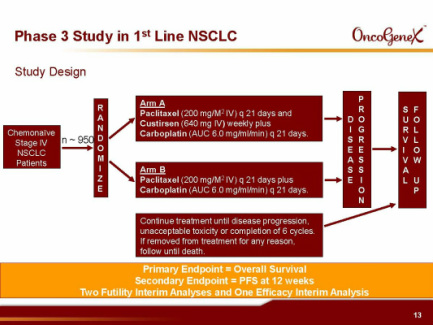
- Plan p3 in combination with 1st-line chemotherapy for advanced NSCLC (950 pts [higher than 700x first planned, w/ 1st line chemo [carboplatin and paclitaxel], run by teva, OS endpoint, two futility and one interim analysis planned). Expected to initiate 2h2011 after manufacture of add'l drug material and performing phase 1 drug-drug interaction (DDI) study, has slipped from early 2011
- Phase 1 trials established the recommended dose in combination with docetaxel (two different treatment schedules), gemcitabine and platinum based chemotherapy (published Clinical Cancer Research 2008), and hormone ablation therapy (published JNCI 2005). Highest dose of 640 mg was well-tolerated
- Five p2 trials, including (294 pts combined in all p1/2 trials; see data figures below):
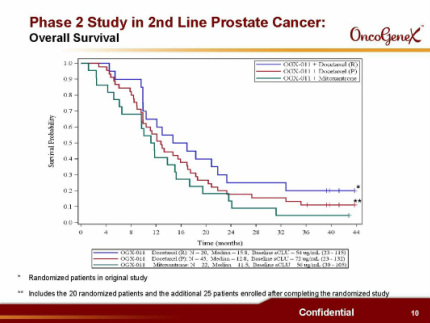
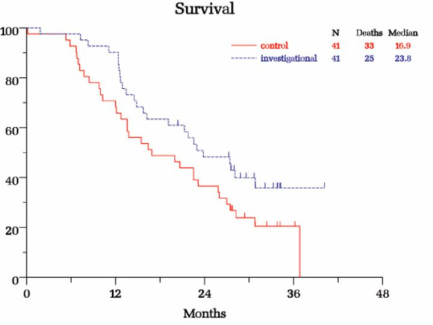
B) Prostate cancer randomized trial in combo with 2nd-line chemotherapy (42 pts who failed 1st-line docetaxel were randomized to OGX-011+ docetaxel retreatment [n=20] or OGX-011+mitoxantrone [n=22], then 5/2007 amended to enroll 25 more pts in docetaxel arm). All pts followed for 36 months or until death (last survival update 8/2010). Pts on docetaxel arm OS 15.8 months for first cohort, 12.8 months overall [2nd cohort had higher serum clusterin levels and poorer prognostic factors], pts on mitoxantrone arm OS 11.5 months, compared to OS of 10 months for pts in TAX 327 study receiving identical chemo regimens [but use caution when comparing any data to historical results]. Impressive 59% pain palliation results on docetaxel arm, w/ 81% of these being durable for >12 weeks (also 46% pain palliation on mito arm). Cabazitaxel TROPIC p3 study showed <10% pain palliation achieved for cabaz or mito. Lower clusterin levels correlated with longer survival (expansion cohort has poorer baseline characteristics including higher clusterin levels). This trial is foundation for phase 3 SATURN trial. Data presented at ASCO GU 2008 and manuscript in preparation.
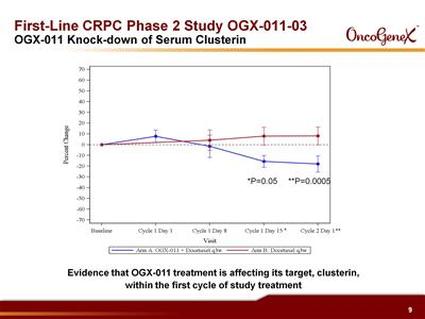
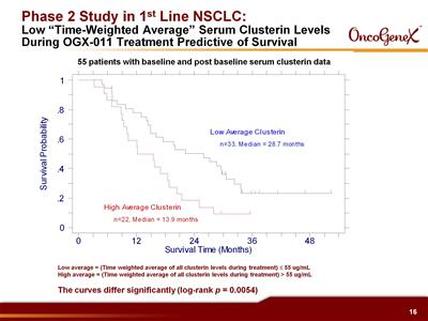
C) Advanced NSCLC open-label trial in combo w/ 1st-line gemcitabine+cisplatin or carboplatin (81 pts, 81% were stage IV, 16% squamous, median followup has been 41 months). As of 4/2009 data release: 14.1 month median OS, 54% and 30% survival at 1 and 2 yrs, respectively [compare to historical results from 5 other studies at 8-10.8 months OS and 33-43% 1 yr survival]. 69% disease control rate. Tx reduces clusterin levels and the degree of this correlates with survival. 2 year survival data presented 2/2009 and manuscript in preparation.
D) Advanced breast open-label trial in combo w/ 1st or 2nd-line docetaxel (5 PR among 15 pts). Published 1/2009 in Clinical Cancer Research
E) Localized prostate cancer in combo w/ hormone ablation prior to prostatectomy. 3 month treatment. Detected drug in tissue samples, along w/ reduce clusterin levels and increased apoptosis. Results at ASCO GU 2008.
Early Stage Clinical Trials:
B) Prostate cancer randomized trial in combo with 2nd-line chemotherapy (42 pts who failed 1st-line docetaxel were randomized to OGX-011+ docetaxel retreatment [n=20] or OGX-011+mitoxantrone [n=22], then 5/2007 amended to enroll 25 more pts in docetaxel arm). All pts followed for 36 months or until death (last survival update 8/2010). Pts on docetaxel arm OS 15.8 months for first cohort, 12.8 months overall [2nd cohort had higher serum clusterin levels and poorer prognostic factors], pts on mitoxantrone arm OS 11.5 months, compared to OS of 10 months for pts in TAX 327 study receiving identical chemo regimens [but use caution when comparing any data to historical results]. Impressive 59% pain palliation results on docetaxel arm, w/ 81% of these being durable for >12 weeks (also 46% pain palliation on mito arm). Cabazitaxel TROPIC p3 study showed <10% pain palliation achieved for cabaz or mito. Lower clusterin levels correlated with longer survival (expansion cohort has poorer baseline characteristics including higher clusterin levels). This trial is foundation for phase 3 SATURN trial. Data presented at ASCO GU 2008 and manuscript in preparation.
C) Advanced NSCLC open-label trial in combo w/ 1st-line gemcitabine+cisplatin or carboplatin (81 pts, 81% were stage IV, 16% squamous, median followup has been 41 months). As of 4/2009 data release: 14.1 month median OS, 54% and 30% survival at 1 and 2 yrs, respectively [compare to historical results from 5 other studies at 8-10.8 months OS and 33-43% 1 yr survival]. 69% disease control rate. Tx reduces clusterin levels and the degree of this correlates with survival. 2 year survival data presented 2/2009 and manuscript in preparation.
D) Advanced breast open-label trial in combo w/ 1st or 2nd-line docetaxel (5 PR among 15 pts). Published 1/2009 in Clinical Cancer Research
E) Localized prostate cancer in combo w/ hormone ablation prior to prostatectomy. 3 month treatment. Detected drug in tissue samples, along w/ reduce clusterin levels and increased apoptosis. Results at ASCO GU 2008.
- Phase 1 trials established the recommended dose in combination with docetaxel (two different treatment schedules), gemcitabine and platinum based chemotherapy (published Clinical Cancer Research 2008), and hormone ablation therapy (published JNCI 2005). Highest dose of 640 mg was well-tolerated
- Five p2 trials, including (294 pts combined in all p1/2 trials; see data figures below):
B) Prostate cancer randomized trial in combo with 2nd-line chemotherapy (42 pts who failed 1st-line docetaxel were randomized to OGX-011+ docetaxel retreatment [n=20] or OGX-011+mitoxantrone [n=22], then 5/2007 amended to enroll 25 more pts in docetaxel arm). All pts followed for 36 months or until death (last survival update 8/2010). Pts on docetaxel arm OS 15.8 months for first cohort, 12.8 months overall [2nd cohort had higher serum clusterin levels and poorer prognostic factors], pts on mitoxantrone arm OS 11.5 months, compared to OS of 10 months for pts in TAX 327 study receiving identical chemo regimens [but use caution when comparing any data to historical results]. Impressive 59% pain palliation results on docetaxel arm, w/ 81% of these being durable for >12 weeks (also 46% pain palliation on mito arm). Cabazitaxel TROPIC p3 study showed <10% pain palliation achieved for cabaz or mito. Lower clusterin levels correlated with longer survival (expansion cohort has poorer baseline characteristics including higher clusterin levels). This trial is foundation for phase 3 SATURN trial. Data presented at ASCO GU 2008 and manuscript in preparation.
C) Advanced NSCLC open-label trial in combo w/ 1st-line gemcitabine+cisplatin or carboplatin (81 pts, 81% were stage IV, 16% squamous, median followup has been 41 months). As of 4/2009 data release: 14.1 month median OS, 54% and 30% survival at 1 and 2 yrs, respectively [compare to historical results from 5 other studies at 8-10.8 months OS and 33-43% 1 yr survival]. 69% disease control rate. Tx reduces clusterin levels and the degree of this correlates with survival. 2 year survival data presented 2/2009 and manuscript in preparation.
D) Advanced breast open-label trial in combo w/ 1st or 2nd-line docetaxel (5 PR among 15 pts). Published 1/2009 in Clinical Cancer Research
E) Localized prostate cancer in combo w/ hormone ablation prior to prostatectomy. 3 month treatment. Detected drug in tissue samples, along w/ reduce clusterin levels and increased apoptosis. Results at ASCO GU 2008.