- ALNY released 2q2011 results on 8/1/11 and held a conference call/webcast (click here for press release). My complete notes from the event can be found below after the jump. Lots of discussion (surprisingly to me) around the TTR program and management provided much color that I will use to update the ALNY research page.
- 1h2011: launched 5x15 strategy and are focusing on the "rapid advancement of RNAi therapeutics that really matter" and believe this will also be a value driver for shareholders
- Recent Highlights/Intro:
- PCS program- advances 2nd generation delivery platform into clinic
- VSP phase 1 data: well tolerated, demonstrated activity and RNAi mechanism of action in pts
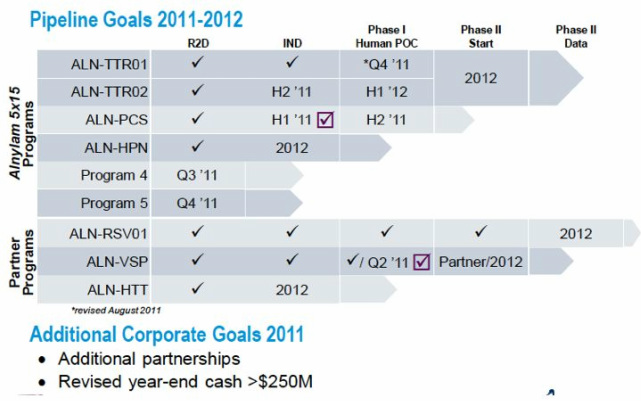
- 2h11: TTR p1 data 4q (slip in timeline from 3q), TTR02 (followon) IND, PCS p1 data
- fully aware that clinical data is key for field of RNAi
- pleased w/ efforts, especially w alnylam biotherapeutics arm (but no details provided)
- Pipeline Updates:
1) TTR
-enrolling in p1 trial
-to accommodate enrollment trends in summer months at EU sites, now expct data 4q11, which will be presented at conference 11/20-22 in japan. Also feel this is the best venue for expected important data for this disease
- follow-on 2nd generation TTR-02 will file IND or equivalent 2h11, completed large # of GLP studies 2q11 for this candidate
2) PCS
-"proprietary" [unless you ask Tekmira] second-generation MC3 lipid.
-Will be UK p1 in subjects w/ elevated cholsterol. 5 cohorts ascending with doses of 0.015 to 0.25 mg/kg. Will focus on safety, PK, measure PCS levels, and serum cholesterol levels
3) HPN addl preclinical data will be presneted 2h11
4) and 5) on target to designate 2 of several preclinical programs (see further discussion below during q&a session) in 3q11 and 4q11, repectively
"Partner" Programs
- VSP: 7/11 pts at recommended p2 dose 1 mg/kg had SD or PR. Have now completed dosing, and 5 pt w/ disease control are continung under an extension protocol
- RSV - enrolling p2b study, on track for 2012 data (see further discussion re interim analysis below in q&a session notes)
- HTT - advancing toward clinical trials (on the slow track though, guides for IND 2012 but no mention of when p1 trial starting)
- Now will have 4 RNAi therapeutics in clincial developent
- cash $316m 6/30/11. Will continue to prudently invest in pipeline. Now expect >$250m cash balance ye2011 (previous guidance was >$275m)
- r&d exp $25m in 2q11 (down $3m y/y), g&a $8.4m
- 2q- progess on delivery collaborations
- extended UBC/Alcana deal thru 7/2012 - this led to discovery of 2nd gen LNPs including MC3
- Precision Nanosystems. lipid nanoparticles. "small LNPs" w/ microfluidics technology. 20nm size gives potential to broaden distribution to targets outside the liver
- Pleased w/ additional ongoing discussions, especially around the application of RNAi to biopharmaceutical manufacturing (this effort - see details on ALNY page - was mentioned over an over in passing on this CC, I am expecting a deal of some sort to be announced 2h2011)
- Regulus/GSK, designated 3rd of 4 targets in collaboration, triggering preclinical milestone.
- Goals for next 12-18 months slide 14 (see below)
- HPN programs goal to "advance towards clinic"
- Aim to form additional partnerships
1)
-What kind of data will be included in the TTR presentation? Full accounting of safety and tolerability, PK/PD, TTR levels (mutant and wt). Obviously safety is most important (encouraged so far, also know that is common LNP technology as shown to be safe in VSP trial).
-Statistical data? No stat powering of p1 studies. In data released in June- TTR levels tightly controlled, so if see KD, should be evident.
next study? all comers vs particular myoptahy? a little ealry. next goal is ttr2 filing adintial studies. end of 2012 advance best produt in pgm into advanced deelopent
02 pot advantages? 2nd gen LNP, 10x inc potency and braodr ther index.
will be picking ONE as go forward pgm. closer aligned in timeline of developt than you hink. goal p2 in 2012.
2)
-Assuming the goal is to move TTR into younger children (prevent accumulation over time). What sort of increased safety hurdle do you expect? You may be confused...not sure younger population is where we are going. Generally not diagnosed/symptomatic until middle age. Goal is to treat pts that are symptomatic- delay progression/disability or show evidence of regression. Life cycle plan would be to eventually get into pre-symptomatic population (but still adults)
- The increased spend, where exactly will that be? What programs? This reflects investment in 5x15 pgms. 2h total R&D expenses will approximate the first half, but lumpy quarter to quarter.
3)
-Re financial guidance - will G&A stay similar? Again, will be lumpy but 2h similar to 1h.
-Milestones or business development factored in for 2h? Expecting modest such contributions in 2h, but not big impact on guidance
-5x15 programs #4 and 5- how close to clinic vs far out like Huntington's program? Have around 6 such programs ("advanced in discovery...behind the scenes" and have done animals work (efficacy and safety ranging from rodents to nonhuman primates) for several. Upon candidate designation, can expect clear evidence of target, disease, devel plan, and, shortly thereafter animal data and filing plan.
- Will TTR02 go forward decision depend on seeing KD w/ TTR01? No, but reiterated that they are expecting good data with TTR01.
-PCS program, when should we expect green light to enroll? Review times are typically a couple of months [note this is longer than in US where the FDA has only 30 days to respond re an IND filing]. We will let public know when initiate. This study should go more rapidly because healthy volunteers [albeit with high cholesterol], not disease pts. Have had conversations w/ regulators, and they expect timely review
4)
- Why the TTR data delay? Is it enrollment issues or need for a higher dose? The issue is enrollment in the summer months, especially at EU sites that made it necessary to delay data presentation until 4q. Also received an invitation to present at this specific meeting [International Symposium on Familial Amyloidotic Polyneuropathy being held November 20 - 22, 2011 in Kumamoto, Japan]
- An update on the RSV trial? We amended the protocol to increase enrollment, up to a max of 120 pt, enrollment is now exceeding expectations. The interim analysis will happen 2h2011 (consisting solely of an adaptive sample size calculation - no futility, efficacy stopping, no statistical penalty) and ALNY will be blinded to results. ALNY will get guidance to either enroll , the 120 pts or add X number of additional pts ...regardless will have results in 2012.
-Increased cash burn...where is it going? We had in mind what $$ it would take to start 5x15, now think we should be investing more based on progress...so largely will be on r&d side. Not any one program responsible. Also progress on conjugate delivery far excceeded expectation, so advancing this effort further
- Comment on Merck closing San Francisco RNAi facility? - This was no surpise on our part [clearly intimated they new of this in advance due to close connections among RNAi community]. It appears as though this was a site shutdown [beautiful 50 person site in SF sticks out like sore thumb], but MRK will still work on RNAi elsewhere (this despite >10k job loss companywide)...so they are clearly still interested in the field, as evidenced by the fact that they are still "joyfully opposing our IP"
5)
-PCS followup. We anticipate reporting reductions in PCSK9 levels and LDL cholesterol, as well as HDL (this metric has been negatively affected by PCSK9 antibodies). Will have data by year's end, but not by AHA in November (ALNY won't be there)
6)
-Delivery question - what is the likelihood of novel (ie, newer than 2nd generation LNP formulation) for 5x15 programs #3,4, and 5? It is pretty likely that we will always be using the best we have. We have improved on MC3. But there is value/comfort in using exisitng formulations too. Excited about conjugate work for sub-cutaneous dosing. Conjugate program will certainly be moving into development. Molecular, rodent, non-human primate safety data in hand already. -Question re Cubist $CBST opt-in for RSV program? -Cubist has months after data release to opt in at slight premoum to the money ALNY has invested. We think they will remain a partner if data is good. This trial is meant to confirm exciting findings in small p2a study. This study will either confirm the data or we'll move on. Right now over 80 pts enrolled, if not expanded. Now in Southern hemisphere RSV season, so no Northern recruitment, but South is not as robust for accrual. Now have over 30 sites globally.
-TKMR litigation effect on Alcana/UBC relatinships? Has no impact.
7)
-VSP partnering update? Some discussions ongoing. Kicked off talks after ASCO, so 6 wks or so in the process. Poster was swamped with Drs and pharma interest
8)
RSV interim outcome announcement? Probably will announce if told to enroll more pts at interim. ie, more vs stop enrollment. Is a 6 month endpt, so data will be in 2012 regardless

 RSS Feed
RSS Feed
