- I weighed in on Amarin $AMRN and its triglyceride lowering drug AMR101 in a recent blog post about the chances of the FDA granting new chemical entity (NCE) status. Another hot topic of discussion and a recent reason for a drop in the value of AMRN shares is concerns over intellectual property protection for the drug (or more precisely, the lack thereof)
- I am assembling some more info on AMRN here, plus you can check out all of my blog posts about the company here.
- A big hat tip to @IMHO_DoYourDD for fun email conversations about AMRN and hard work assembling blog posts on Amarin's IP and stock prospects
- In this first post (see below), I'll look at the patents already issued to Amarin.
Amarin Patents (via acquisition of Laxdale in 2004):
7,119,118: "Highly purified ethyl EPA and other EPA derivatives for treatment of huntington's disease"
6,689,812: "Highly purified ethyl EPA and other EPA derivatives for psychiatric and neurological disorders"
6,384,077: "Highly purified EPA for treatment of schizophrenia and related disorders"
All three of the above patents make claims only for use in treating various neurological disorders and have no applicability to the current use of ethyl-EPA (AMR101) for cardiovascular disease.
6,479,544: "Therapeutic combinations of fatty acids"
This is the patent that the company supposedly claims currently provides protection for AMR101. While I am by no means a patent expert, I find this claim troubling - and would obviously feel much better if Amarin succeeds in gaining the issuance of additional patent(s) around AMR101 (which I do feel is likely in the next year).
The key claim in this patent is:
1. "A pharmaceutical composition comprising a combination of a) a biologically assimilable eicosapentaenoic compound (EPA) having a purity of at least 90% with b) a biologically assimilable arachidonic compound (AA) or a precursor thereof having a purity of at least 90%."
I'll mention just a couple additional claims that I find relevant:
2. A pharmaceutical composition according to claim 1 in which the ratio of EPA to AA or a precursor thereof is between 1:1 and 20:1.
6. A pharmaceutical composition according to claim 1 which consists essentially of a combination of (a) and (b)
14. A method of treating or preventing a disease which comprises administering an effective amount of a composition according to claim 1 to a subject prone to or afflicted with such disease, and wherein the disease is an amenable disease selected from the group consisting of: asthma or other respiratory disease; a disease of the gastrointestinal tract; an inflammatory disease; a cardiovascular disease; a dyslipidaemia, diabetes or other form of metabolic disease; a dermatological disease; a kidney or urinary tract disease; a liver disease; a disease of the male or female reproductive system or related secondary sexual organs; a cancer; a disease of the head or neck; and an infection caused by a virus, bacterium, fungus, protozoa or other organism.
15. A pharmaceutical composition according to claim 1 wherein its sole active ingredient consists essentially of the combination of (a) with (b)
Arachidonic acid (AA) is a metabolite of omega-6 fatty acids (in contrast EPA is an omega-3). AA itself is the precursor for inflammatory molecules known as eicosanoids. EPA competes with AA in the body and taking EPA has actually been shown to reduce AA levels. AA is generally described as being found in fatty red meat, liver, and egg yolks.
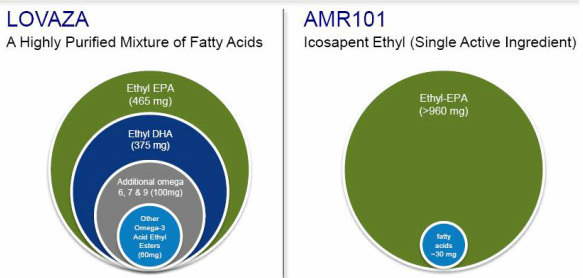
It is unclear exactly how much AA is present in AMR101. But the one thing that is certain is the answer is a minute amount. One over-the-counter 70% EPA fish oil product is described as containing "a tiny amount" of omega-6, which could include AA. More simply, based on the statements that AMR101 is >96% pure ethyl-EPA, the product cannot theoretically contain any more than 4% AA. AA therefore can't be present in any more than a 1:24 ratio to EPA (4/96), which falls outside the range of claim 2 above.
While claims 1 and 6 don't require a particular ratio of EPA and AA, I don't think these claims will stand up. To claim patent protection, one would have to call AMR101, a highly purified (remember, they want EPA to be considered as a NCE compared to less purified fish oil, Lovaza) EPA product, consisting of what amounts to a trace byproduct of AA, to be a combination of EPA and ">90% purified AA."
I'll look at pending, published patent applications in another post. Several of these could end up protecting AMR101 until 2030 or beyond. But keep it mind when management or AMRN bulls talk about "an issued composition patent providing protection through 2021"...this is the patent they mean, and it doesn't offer a whole lot to stand on.
Disclosure: I own shares of AMRN.
Please consider a small contribution in support of the site if you find the content on BiotechDueDiligence to be valuable.
 RSS Feed
RSS Feed
