PRESENTATION TYPE: Oral or Poster
CURRENT CATEGORY: Portal Hypertension and Other Complications of Cirrhosis
CURRENT DESCRIPTORS: Q03. Portal Hypertension: Experimental
TITLE: Albumin and MELD score predict decompensation in patients with HCV cirrhosis and thrombocytopenia on interferon therapy: analysis from the ENABLE studies
AUTHORS (FIRST NAME, LAST NAME): Nezam Afdhal1, Edoardo G. Giannini2, Samuel Sigal3, Stuart C. Gordon4, Gregory T. Everson5, Abdullah M. Al-Osaimi6, Geoffrey M. Dusheiko7, Teresa Casanovas8, Norbert Brau9, Sandra Y. Vasey10, Malini Iyengar10, Ulla Forssen10, Fiona M. Campbell11, Dickens Theodore12
Institutional Author(s):
INSTITUTIONS (ALL): 1. Hepatology, Harvard Medical School, Boston, MA, United States.
2. Universita' degli Studi di Genova, Genova, Italy.
3. NYU Langone Medical Center, New York, NY, United States.
4. Henry Ford Health System, Detroit, MI, United States.
5. The Children's Hospital/University of Colorado Health Science Center, Denver, CO, United States.
6. UVA Health System, Charlottesville, VA, United States.
7. Royal Free Hospital, London, United Kingdom.
8. Ciudad Sanitaria y Universitaria de Bellvitge, Barcelona, Spain.
9. Bronx VA Medical Center, Bronx, NY, United States.
10. GlaxoSmithKline, Upper Providence, PA, United States.
11. GlaxoSmithKline, London, United Kingdom.
12. GlaxoSmithKline, Research Triangle Park, NC, United States.
INTRO: Peginterferon (IFN)-based treatment of patients with compensated hepatitis C virus (HCV) cirrhosis has been associated with the development of hepatic decompensation (reported as Grade 3/Grade 4 adverse events) in up to 5% of patients after 16 weeks of treatment and a high rate of serious adverse events (>45%) (CUPIC Trial, Hezode, EASL 2012).
AIMS: To evaluate risk factors for hepatic decompensation during IFN-based therapy in cirrhotic patients with compensated disease and thrombocytopenia (platelet count <75,000/µL) enrolled in the 2 ENABLE trials.
METHODS: 1439 patients who received treatment with IFN and ribavirin in combination with eltrombopag (n=955) or matched placebo (n=484) for 24 or 48 weeks according to viral genotype were analyzed for clinical hepatic decompensation events by an independent expert panel. Endpoints included all clinical liver events and mortality during treatment and 30 days follow-up. Change in Child Pugh (CP) score from baseline until 30-day follow-up was also recorded.
RESULTS: The median age was 52 years, 63% were male, 74% were white, 64% were genotype 1, and 95% were CP A. The median baseline platelet count was 59,500/µL and 78% had elevated ALT at baseline. 160 patients, 35 (7%) patients on placebo and 125 (13%) on eltrombopag, had a total of 252 hepatic decompensation events. In multivariate analysis, risk factors for decompensation included the baseline characteristics: age (≥60 vs 50-<60 vs <50), MELD (≥10 vs <10), albumin (≤3.5 g/L vs >3.5 g/L), ALT (<3 x ULN vs ≥3 x ULN), bilirubin (>0.7 mg/dL vs ≤0.7 mg/dL), and history of alcohol abuse (yes vs no). Utilizing the MELD and albumin cutoffs the odds ratio (95% CI) of hepatic decompensation was 1.95 (1.27, 2.99) and 2.13 (1.42, 3.18), respectively. The exposure adjusted incidence rates for decompensation (95% CI) were 13.3/100 patient years (PYs) (8.9, 17.7) for placebo, and 19.9/100 PYs (16.4, 23.4) for eltrombopag. Ascites and encephalopathy were more common in the eltrombopag group. An increase in CP score of >2 occurred in 6% of patients on placebo and 8% on eltrombopag. In this subset of patients with hepatic decompensation, 10% on eltrombopag and 3% on placebo achieved sustained virologic response.
CONCLUSION: In patients with compensated cirrhosis with thrombocytopenia, IFN-based therapies increase the risk of hepatic decompensation. MELD score and albumin can be used to stratify risk vs benefit of therapy.
PRESENTATION TYPE: Oral or Poster
CURRENT CATEGORY: Viral Hepatitis C
CURRENT DESCRIPTORS: S07. HCV: Treatment
TITLE: Improved SVR in a unique cohort of non-cirrhotic thrombocytopenic patients with hepatitis C virus (HCV) treated in the global multicenter ENABLE 1 and 2 trials
AUTHORS (FIRST NAME, LAST NAME): Nezam Afdhal1, Edoardo G. Giannini2, Samuel Sigal3, Andrew J. Muir4, K. Rajender Reddy5, I-Shyan Sheen6, Shanthi Vijayaraghavan7, Magdy Elkashab8, Manuel Romero-Gomez9, Geoffrey Dusheiko10, Malini Iyengar11, Sandra Y. Vasey11, Fiona M. Campbell12, Dickens Theodore13
Institutional Author(s):
INSTITUTIONS (ALL): 1. Hepatology, Harvard Medical School, Boston, MA, United States.
2. Universita' degli Studi di Genova, Genova, Italy.
3. NYU Langone Medical Center, New York, NY, United States.
4. Duke University Medical Center, Durham, NC, United States.
5. Trustees of the University of Pennsylvania, Philadelphia, PA, United States.
6. Chang Gung Memorial Hospital, Taipei, Taiwan.
7. Lifeline Multispecialty Hospital, Chennai, India.
8. Toronto Liver Centre, Toronto, ON, Canada.
9. Hospital Nuestra Senora de Valme, Sevilla, Spain.
10. Royal Free Hospital, London, United Kingdom.
11. GlaxoSmithKline, Upper Providence, PA, United States.
12. GlaxoSmithKline, London, United Kingdom.
13. GlaxoSmithKline, Research Triangle Park, NC, United States.
ABSTRACT BODY: Thrombocytopenia can be due to HCV cirrhosis or, less commonly, HCV via immune mediated reduction in platelets. The true prevalence and characteristics of non-cirrhotic thrombocytopenia have not been described.
AIMS: To evaluate the prevalence, clinical features, and treatment outcome of non-cirrhotic thrombocytopenia and cirrhosis-related thrombocytopenia in patients with platelets <75,000/µL enrolled in the ENABLE studies, which assessed the impact of eltrombopag (ELT) on achieving SVR in thrombocytopenic patients who were otherwise ineligible for HCV antiviral therapy.
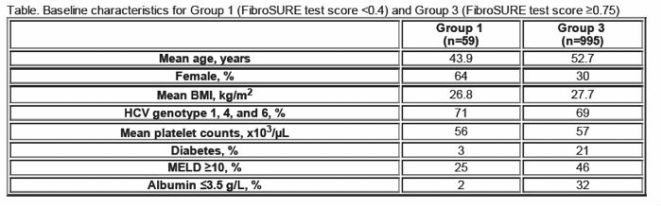
METHODS: 1258 patients from the ENABLE studies were divided into 3 groups based on baseline FibroSURE (FS) results: Group 1 Non-cirrhotic (n=59) FS <0.4; Group 2 Indeterminate (n=214) FS 0.4-<0.75; Group 3 Cirrhotic (n=995) FS ≥0.75. Demographics and outcomes of Groups 2 and 3 were similar, but Group 2 was excluded from this analysis since FS was indeterminate for cirrhosis.
RESULTS: Baseline characteristics are presented in the Table. At every time point virologic response was better in Group 1 than Group 3: EVR 76% vs 55%; ETR 69% vs 25%; SVR 42% vs 17%. Virologic responses were superior at all time points in both groups for patients treated with ELT compared to placebo (PL), including at SVR (Group 1: ELT 56%, PL 32%; and Group 3: ELT 18%, PL 12%). In the safety analysis, 1 patient (2%) in Group 1 had hepatic decompensation vs 116 patients (12%) in Group 3. Thromboembolic events were seen in 3% of patients in both groups.
CONCLUSION: We describe for the first time a demographically and clinically unique population from the ENABLE 1 and ENABLE 2 trials with thrombocytopenia for reasons other than cirrhosis. Compared to patients with cirrhosis-induced thrombocytopenia this non-cirrhotic patient population 1) was predominantly female and younger, 2) had superior virologic responses at all time points, and 3) had a lower incidence of hepatic decompensation.
 RSS Feed
RSS Feed
