Related Content:
- ISIS Stock Research homepage
- Index of all ISIS-related BiotechDueDiligence blog posts
- Chimera blog post: updates on ISIS and AEGR after FDA Advisory Committee meetings
- ISIS notes and slides from 2013 JP Morgan conference
- AEGR Notes and slides from FDA Advisory Committee meeting and briefing document
(Continue reading for full post)
- first systemic antisense drug approved
- These HoFH patients have LDL 500-1000. 20 year old has arteries of an 80 year old. Before statins, died in teens - now has improved to 30s
1. simple, weekly sub-cutaneous (SC) injection at home. size of insulin needle. "no daily reminder of disease" [my sense is the typical patient would rather than an oral drug vs. give themselves a weekly shot and am aware of no market research to the contrary]
2. only need standard heart healthy diet vs low fat/nutritional counseling/vitamin supplementation - meaningful to young/active population [On the other hand, AEGR CEO stated that the diet was so easy, he requested every Aegerion employee to follow it]
3. drug interactions - many HoFH patients take >12 meds per day [According to the JUXTAPID label, lomitapide is an inhibitor of and is metabolized by CYP3A4. The drug cannot be used with moderate or strong CYP3A4 inhibitors and must be used at a lower dose in combination with weak CYP3A4 inhibitors. Dosing of warfarin and simvastatin must be reduced, but not other statins, fenofibrates, niacin, or ezetimibe. The notion that these drug-drug interactions will determine the physician's choice between mipomersen and lomitapide in a severe population that already has a complex medication profile strains credulity in my opinion.]
Sales Launch
- Genzyme will leverage their CV salesforce to target lipid specialists
- Genzyme: "we are ready to launch"
- we won't provide a target # of patients on drug this year like AEGR
- we will share KYNAMRO price at launch [since revealed as $176k per patient per year] , consistent with apheresis
- data for patients under 18 years not in label (though we had some on trial). We will further study this population [This was not surprising in hindsight but has to be a disappointment for ISIS. They get no credit for a "modest" subpopulation of the patients, 10-15% if I recall. The label reads as though no children were enrolled, as was the case for the lomitapide trial]
- Our first task to identify patients and refer to lipidologist
- no real difference between ISIS and AEGR REMS. Both to assure long-term liver safety
Question and Answer session
This is a great outcome here with the FDA, obviously not as good as the first outcome in Europe. I was wondering if you had any insights into what went different with the two processes, what the EMA might be failing to appreciate at this point that the FDA did appreciate?
We do, but we’re not going to discuss that today. We’re in the midst of the re-examination in Europe. We are very, very optimistic that that will successful. And once their process is concluded then we may be able to discuss some of the specific differences and different concerns and so on in more detail, but certainly not today.
What is the length of time from prescription to product out door?
4-6weeks, could be less. depends on which managed care company. There are patients who have not been referred to lipidologists, and we can reach them because we are part of Sanofi. Cardiologist ID's them, but may still refer to lipidologist for counseling
Label states patients should come off drug after 6 months if not enough efficacy. What percentage of patients was that in clinical trials?
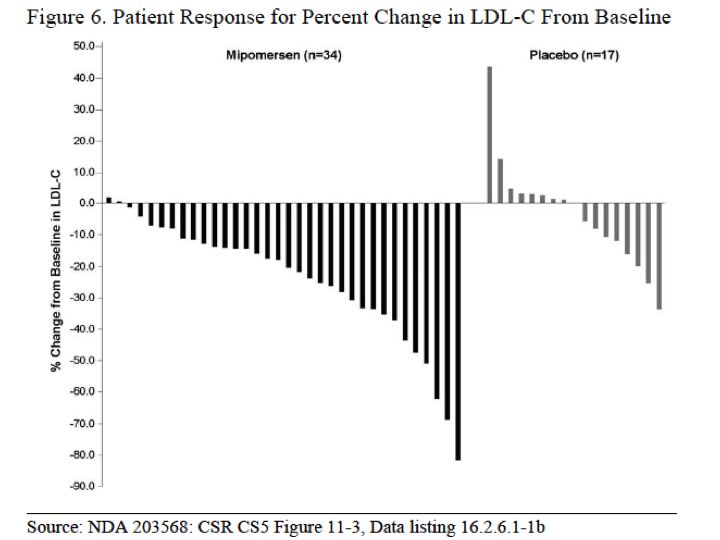
"very small" though there was more variabiltiy in response in HoFH vs other populations. We learned that "low responders" are really "slow responders" [This is utter BS. The guidance in the KYNAMRO label is "after initiation of KYNAMRO therapy lipid levels should be monitored at least every 3 months for the first year. Maximal reduction of LDL-C may be seen with KYNAMRO therapy after approximately 6 months (based on the time to steady state seen in clinical studies). Health care providers should assess the patient's LDL-C level after 6 months to determine if the LDL-C reduction achieved with KYNAMO is sufficiently robust to warrant the potential risk of liver toxicity" If you look at the waterfall plot below, 20% (7/34) patients failed to achieve a 10% reduction at 6 months. If you use the threshold of 15% proposed by the FDA Medical Officer, it appears as though up to 40% (14/34) of patients would have met the discontinuation criteria. Of course a particular threshold is NOT stated in the label and it would remain at the discretion of the lipidologist. There is no such discontinuation suggestion in the JUXTAPID label]
We won't quantify that. Expenses are shared until profitability, then Genzyme pays. We will issues general company financial guidance at our yearend call
PMC also has anti-dsDNA antibody monitoring? Is this due to an imunogenicity concern?
This is based on a theoretical concern. FDA wants us to look in more patients and develop more sensitive assays
 RSS Feed
RSS Feed
