Navigate the Ligand Pharmaceutials, Inc. ($LGND) Stock Research Pages
- Introduction (valuation, financials, ownership, guidance, helpful links)
- Promacta (eltrombopag) (key asset licensed to GSK - platelet booster for many thrombocytopenia conditions)
- Kyprolis (carfilzomib) (key asset licensed to Amgen - developed by ONXX for multiple myeloma)
- Other Marketed Products (description of assets currently generating royalty revenue)
- Late-stage Pipeline Assets (generally partnered and developed at little to no cost to LGND and represent future royalty sources)
- Early-stage Development Programs (internal and partnered, future "shots on goal" or out-licensing opportunities)
- Acquisitions (Cydex, Metabasis ($MBRX), Pharmacopeia ($PCOP), Neurogen ($NRGN) - complete terms and details)
- Captisol-Cydex (additional info on the formulation technology acquired in 2011 and details on partnerships)
- Discontinued Programs (with >90 biotech and pharma assets in development, not everything works out!)
Kyprolis® (carfilzomib) for multiple myeloma
Status: Marketed by Amgen Source: Cydex
- Kyprolis® (carfilzomib) for Injection is a type of medicine called a proteasome inhibitor that works as a single-agent therapy. KYPROLIS is approved for the treatment of patients with multiple myeloma who have already received at least two other treatments including bortezomib and an immunomodulatory agent (lenalidomide and/or thalidomide), and whose disease has progressed (got worse) on their last therapy or within 60 days of their last therapy. Approval is based on how many patients responded to treatment. Improvement in survival or symptoms has not been proven. (Source: www.kyprolis.com)
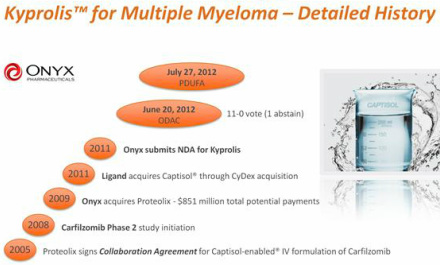
- Selective, "next-generation" proteasome inhibitor developed by Onyx $ONXX (via Proteolix acquisition 11/2009 for $276m plus up to $575m in earnouts). Amgen ($AMGN) aquired Onyx Pharamecuticals, Inc ($ONXX) on October 1, 2013 for $125 per share in cash, or $9.7 billion net of estimated Onyx cash. (Source: Amgen Press Release)
- "The proteasome is a protein complex that exists in all cells, whether healthy or cancerous. The proteasome controls the turnover of proteins in cells in a regulated manner, but cancer cells are more susceptible to cell death when the proteasome is inhibited. Carfilzomib is a novel small molecule, belonging to a class known as peptide ketoepoxides, and is designed to inhibit the proteasome and enable sustained suppression of protein degradation in tumor cells" (2010 ONXX 10k)
- "We are conducting multiple clinical trials evaluating carfilzomib as a monotherapy in relapsed and/or refractory multiple myeloma patients and in combination with other anti-cancer agents and chemotherapies. Multiple myeloma is the second most common hematologic cancer and results from an abnormality of plasma cells, usually in the bone marrow"
- Click here for 3/2011 ONXX corporate slide deck (Protected file so I couldn't post relevant figures)
- Click here for extracted passages from 2010 ONXX 10k related to Carfilzomib
- 10/2005 license and supply agreement with Cydex for access to Clinical and Commercial grade captisol. Click here to access redacted agreement starting on p118 of 2009 ONXX 10k. Terms: Proteolix must buy 100% of Captisol from Cydex; must provide long-range forecast of captisol needs prior to anticipated commerical launch; must provide a rolling quarterly detailed commercial forecast of anticipated product needs; Undisclosed upfront fee; undisclosed clinical/regulatory milestones (6 total); undisclosed sales milestones (3 total), tiered royalties payable quarterly (4 different undisclosed rates); royalties continue on country by country basis until last of licensed patents expires [see list of patents - US patents expired late 2010 unless ONXX switched to the high purity for of Captisol that other partners have] plus an undisclosed additional number of years at a reduced royalty rate; agreed to pricing of captisol- adjustable annually based on inflation
Clinical Trials Info and Data
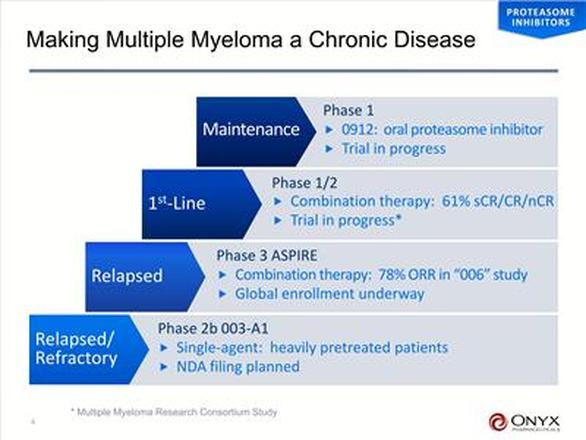
- 6/2010: presented data from phase 1b/2 trial "006" of combo of carfilzomib + lenalidomide and dexamethasone in relapsed MM. Established safety of combo an saw 75% ORR
- 12/2010: positive p2 data from ongoing trial "004" evaluating single-agent carfilzomib in bortezomib (Velcade)-naive pts with refractory MM - 123 pts showed ORR of 53% with minimal neuropathy, median duration of response of >13 months
- 12/2010: positive "003-A1" single-agent p2b trial data presented at ASH meeting (257 pts, 15.5m median OS, 24.1% ORR [primary endpt], w/o worsening neuropathy [77% had grade 1/2 upon entering study], 8.3m median duration of response, pts had median of 5 prior therapies, clinical benefit rate 34.2%). In bortezomib (Velcade) refractory subset (128 pts), ORR was 19% and CBR 31% - Click here for press release
- This study is the basis of the ongoing US FDA NDA filing, received fast track status 1/2011.
- 3/2011: expect rolling NDA filing complete as soon as mid-2011 - could result in accelerated approval and launch in 2012.
- Two large ongoing p3 trials to be completed in 2012:
- "FOCUS" aka "011" EU registrational trial for relapsed/refractory MM started enrolling in 9/2010- randomized to Carfilzomib (20 mg/m2 increasing to 27 mg/m2 after cycle 1 vs best supportive care of low dose steroids plus optional cytoxan. 3/2011: amended trial design [click here for PR] by 1) changing primary endpt to OS [was PFS], and 2) increasing pt enrollment from 84 to 300 pts [will inc enrollment at current sites and add more sites]. This trial will be used in combo with "003-A1" for EU registration, after seeking advice from EMEA 5/2009, Primary endpt is PFS
- "ASPIRE" aka "009" international combination p3 for relapsed MM started enrolling 9/2010 (target 700 pts w/ 1-3 prior treatment regimens). Randomized, open-label of carfilzomib + lenalidomide and low dose dexamethasone vs lenalidomide/dexamethasone alone. Primary endpt is PFS and has SPA with FDA [agreement in 1/2010], plus scientific advice from EMEA. "The ASPIRE trial may either serve as the confirmatory trial for full approval, if accelerated approval is granted on the basis of the 003-A1 data, or would allow for initial approval of the NDA if the trial successfully meets the requirements of the SPA" (ONXX 2010 10k)
- First line MM: phase 1/2 trial ongoing by U. Michigan Cancer Center as of 3/2011: 67% CR with combination therapy. 12/2010 initial data - 100% ORR in newly diagnosed (no prior treatments) MM pts with carfilzomib + lenalidomide and dexamethasone
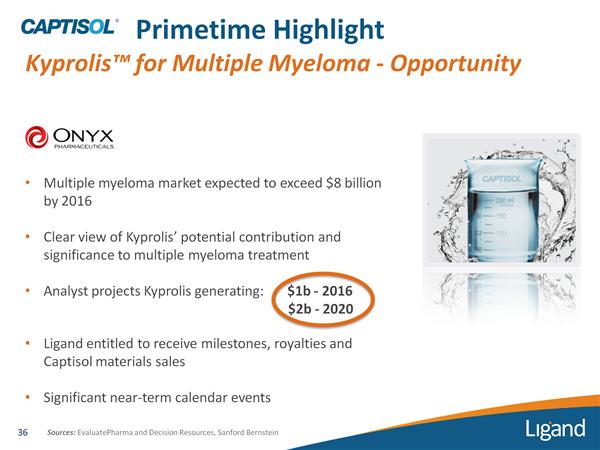
- 9/2010: Ono bought Japan rights $59m upfront (LGND executives described this as "stunning" price). Due milestones, royalties and material sales
- 8/2011: ONXX and Multiple Myeloma Research Fndn launched expanded access program in US for patients with no other treatment options. This effort, considered a clinical trial, will enroll relapsed/refractory MM pts at 40 sites who have failed at least 4 treatment regimens - click here for PR.