Navigate the Reorganized Infinity Pharma $INFI Research Pages
Introduction - General corporate updates, financials, and resources
Saridegib (IPI-926) - Hedgehog inhibitor in multiple phase 2 cancer trials
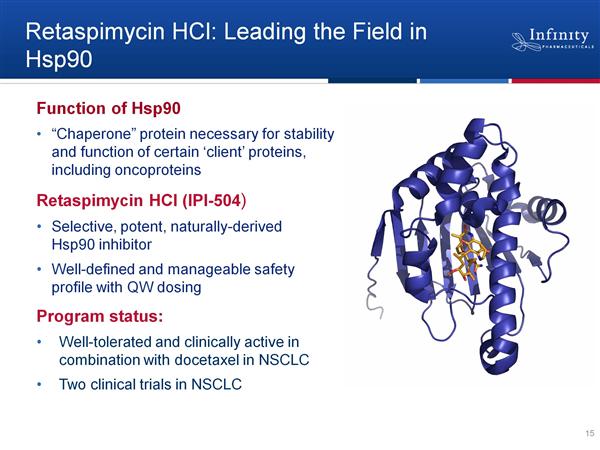
Retaspimycin HCl (IPI-504) - HSP90 inhibitor in phase 2 for lung cancer
IPI-145 - PI3K inhibitor in phase 1 for oncology and inflammatory diseases
Pipeline - including IPI-940 pain drug candidate licensed to Purdue Pharma
Saridegib (IPI-926) - Hedgehog inhibitor in multiple phase 2 cancer trials
Retaspimycin HCl (IPI-504) - HSP90 inhibitor in phase 2 for lung cancer
IPI-145 - PI3K inhibitor in phase 1 for oncology and inflammatory diseases
Pipeline - including IPI-940 pain drug candidate licensed to Purdue Pharma
Retaspimycin HCl (aka IPI-504) HSP90 Inhibitor - Introduction
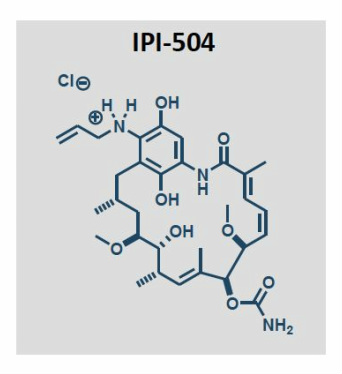
- Retaspimycin hydrochloride, IV-administered small molecule, semi-synthetic analog of natural product geldanamycin, water soluble >200 mg/mL, administer as 30 min infusion.
- 6/2011 CC: preclinical data suggest this drug will work best as combo agent, will focus on difficult to treat NSCLC patient populations
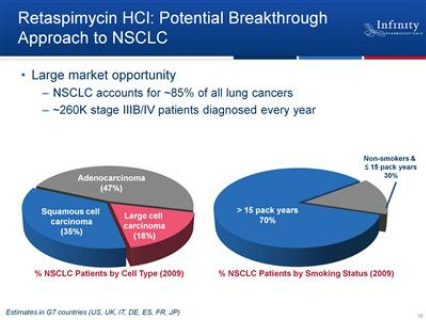
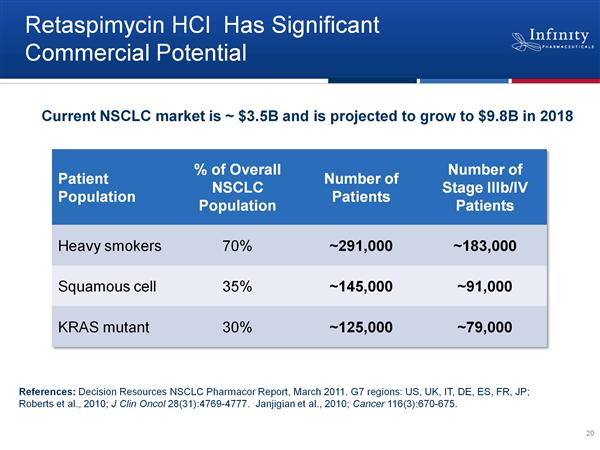
- 200,000 pts per year with stage 3-4 lung cancer
- 6/2011 CC: Company was asked what is mechanism of action of synergy with docetaxel? "We have made clinical discovery, not model-able in lab (related to effect in smoking/squamous subpopulations)...we are trying to build mouse models to explain this effect. [aka, we have no idea!]
- 10 patents on this and related compounds expire 2024-2025 (see structure below).
- 10/24/07 EORTC poster lists MTD from p1 trial as 225 mg/m2. Click here for poster reprint
- 2008 EORTC poster concluded "We find the order of biochemical potency to be NVP-AUY922 >> SNX-2112 ≥ IPI-493 ≥ IPI-504 ≥ BIIB-021" Click here for poster reprint
- 2009 AACR poster- developed assay to study the association between various drugs and HSP90 protein - click here for poster reprint
- 12/2008 rights to IPI-504 and IPI-493 returned by Medimmune (partnered 8/2006 for $70m upfront, $430m milestones while in p1, 50/50 cost sharing)- would owe mid-single digit royalties (6/2011 CC).
Retaspamycin HCL (IPI-504) - Clinical Trials
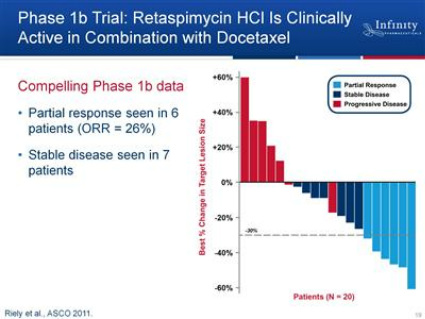
- Phase 1b combo trial w/ docetaxel in advanced NSCLC - COMPLETE
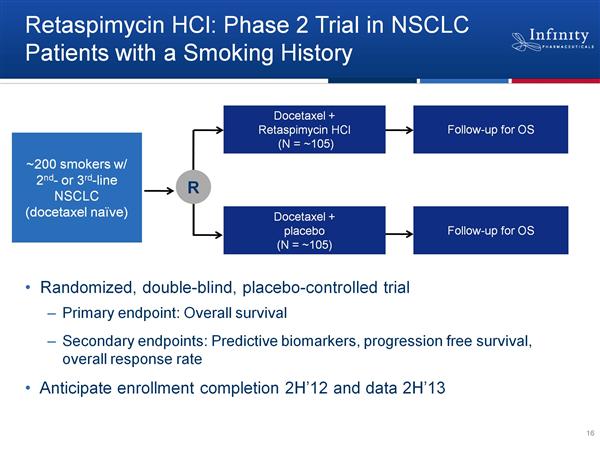
- 5/2011: initiated phase 2 trial of combo w/ docetaxel in NSCLC. (Click for ClinicalTrials.gov listing - Click for PR)
--Preliminary data presented at ASCO 2009 (57 pts total, dose 400 mg/m2 twice weekly for two weeks, off drug third week, overall response rate 7% with all responses seen in EGFR wild-type patients...Click here for poster reprint and click here for a powerpoint slide deck with much of the same data-easier to view).
--Final data presented at ASCO 6/2011 (Click here for PR - Click to download poster): 6 PR in 23 pts, for overall response rate of 26% (6/2011 webcast and CC: docetaxel single agent response rate in NSCLC was 8% and PFS of 3 months), higher in those with squamous cell carcinoma (3/7) or history of heavy smoking (6/18; both of these subgroups have historically poor prognosis). Also 7 additional patients with stable disease (SD). No drug-drug interaction impact on docetaxel clearance. "Notably, this combination was well-tolerated, with no patient showing an increase of liver function tests leading to dose reduction or discontinuation, or reporting significant visual problems." ...6/2011 webcast: no discontinuations for toxicity, no significant ocular toxicity. Note: the poster does not list any ocular side effects at all - this seems sketchy as they were seen in 16% of patients in the single agent trial...maybe they "adjusted" their definitions [why repeatedly say no "signicicant" ocular toxicities vs saying "no ocular adverse events" if really no issue? Or maybe a duration issue, as only 2/23 pts stayed on drug >9 months
--Includes interim analysis - evaluate efficacy in relation to patient characteristics (histology, tobacco exposure, biomarkers) then can be expanded in certain population(s) based on overall response rate. - Click here for PR. [Note they did not restrict the trial to squamous histology and do not seem to be in a hurry to follow up on signal in ALK+ patients]
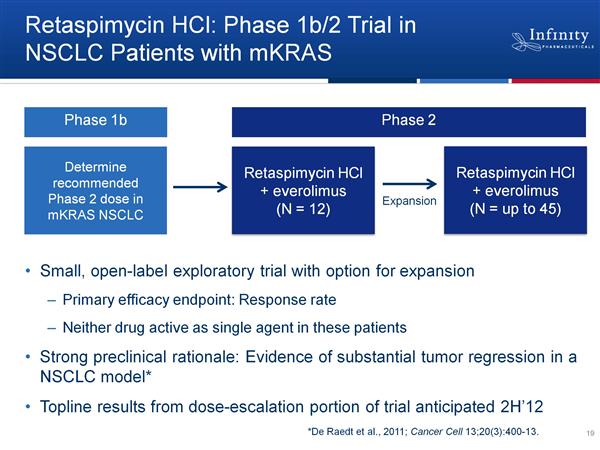
- 8/2011: initiated phase 1b/2 NSCLC combo trial of IPI-504 + Affintor (Click for ClinicalTrials.gov listing)
--8/2011 2q11 CC: Synergy/tumor regression seen in mouse model.
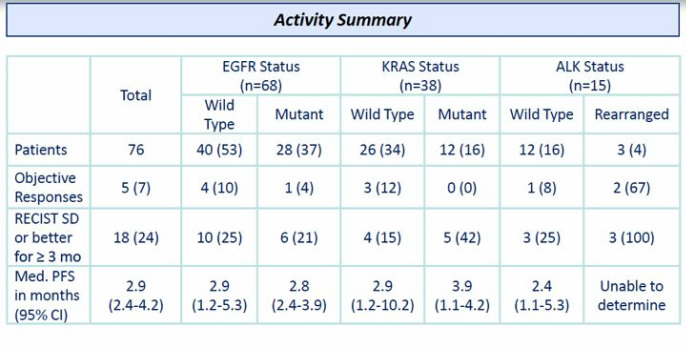
- Phase 2 single agent open-label trial in stage IIIB/IV NSCLC single agent - COMPLETE
--Starting dose was 400 mg/m2, later decreased to 225 mg/m2 based on toxicity in a different study.
--Data was reported at ASCO 6/2010 ( Click here for poster reprint) and published in JCO 2010.
--76 pts, objective RR was 7% (10% EGFR wt, 4% EGFR mut, 12% KRAS wt). 2 PR and 1 SD in 3 pts w/ ALK rearrangement. (see chart below)
--34-56% of patients experienced various liver-related adverse events and 16% exhibited blurred vision. 3 deaths, all rated "possibly related" to IPI-504
- 10/2010 initiated Phase 2 trial in NSCLC pts w/ ALK rearrangement: Investigator sponsored trial at Mass General Hospital to validate above results, plan for 58 pts (Click here for ClinicalTrials.gov listing)
- Phase 2 combo w/ Herceptin (trastuzumab) in HER2+ metastatic breast cancer - COMPLETE
-- Final results presented at ASCO 6/2011 (Click here for abstract): 1 PR and 14 SD (1 over 6 months) among 26 pts treated, no dose-limiting toxicity. Did not meet pre-specified criteria to expand trial.
-- No further development in breast cancer planned
- Phase 3 trial in GIST (initiated 10/2008) - HALTED 4/2009 after only 46 pts because imbalance of deaths - greater in treated arm. Dosing was 400 mg/m2 twice weekly iv for two weeks followed by one week off (Click here for clinicaltrials.gov listing). Previously 5/2008 p1/2 GIST data- 36 pts, single arm (3% PR, 67% SD) led to this phase 3 initiation.